Variables Affecting Shoot Growth and Plantlet Recovery in Tissue Cultures of Drug-Type Cannabis sativa L
- PMID: 34621286
- PMCID: PMC8491305
- DOI: 10.3389/fpls.2021.732344
Variables Affecting Shoot Growth and Plantlet Recovery in Tissue Cultures of Drug-Type Cannabis sativa L
Abstract
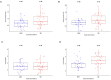
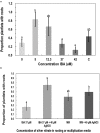
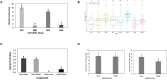
Tissue culture approaches are widely used in crop plants for the purposes of micropropagation, regeneration of plants through organogenesis, obtaining pathogen-free plantlets from meristem culture, and developing genetically modified plants. In this research, we evaluated variables that can influence the success of shoot growth and plantlet production in tissue cultures of drug-type Cannabis sativa L. (marijuana). Various sterilization methods were tested to ensure shoot development from nodal explants by limiting the frequency of contaminating endophytes, which otherwise caused the death of explants. Seven commercially grown tetrahydrocannabinol (THC)-containing cannabis genotypes (strains) showed significant differences in response to shoot growth from meristems and nodal explants on Murashige and Skoog (MS) medium containing thidiazuron (1 μM) and naphthaleneacetic acid (0.5 μM) plus 1% activated charcoal. The effect of Driver and Kuniyuki Walnut (DKW) or MS basal salts in media on shoot length and leaf numbers from nodal explants was compared and showed genotype dependency with regard to the growth response. To obtain rooted plantlets, shoots from meristems and nodal explants of genotype Moby Dick were evaluated for rooting, following the addition of sodium metasilicate, silver nitrate, indole-3-butyric acid (IBA), kinetin, or 2,4-D. Sodium metasilicate improved the visual appearance of the foliage and improved the rate of rooting. Silver nitrate also promoted rooting. Following acclimatization, plantlet survival in hydroponic culture, peat plugs, and rockwool substrate was 57, 76, and 83%, respectively. The development of plantlets from meristems is described for the first time in C. sativa and has potential for obtaining pathogen-free plants. The callogenesis response of leaf explants of 11 genotypes on MS medium without activated charcoal was 35% to 100%, depending on the genotype; organogenesis was not observed. The success in recovery of plantlets from meristems and nodal explants is influenced by cannabis genotype, degree of endophytic contamination of the explants, and frequency of rooting. The procedures described here have potential applications for research and commercial utility to obtain plantlets in stage 1 tissue cultures of C. sativa.
Keywords: Cannabis sativa L.; callogenesis; meristems; micropropagation; nodal explants; plantlet recovery; rooting; shoot growth.
Copyright © 2021 Holmes, Lung, Collyer and Punja.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Barnett S. E., Cala A. R., Hansen J. L., Crawford J., Viands D. R., Smart L. B., et al. . (2020). Evaluating the microbiome of hemp. Phytobiomes J. 4, 351–363. 10.1094/PBIOMES-06-20-0046-R - DOI
-
- Braemer R., Paris M. (1987). Biotransformation of cannabinoids by a cell suspension culture of Cannabis sativa L. Plant Cell Rep. 6, 150–152. - PubMed
-
- Chandra S., Lata H., Khan I. A., ElSohly M. A. (2017). “Cannabis sativa L.: botany and horticulture,” in Cannabis sativa L. Botany and Biotechnology, eds S. Chandra, H. Lata, M. ElSohly M (Cham: Springer; ). 10.1007/978-3-319-54564-6 - DOI
-
- Cheong E. J., Mock R., Li R. (2012). Elimination of five viruses from sugarcane using in vitro culture of axillary buds and apical meristems. Plant Cell Tissue Organ. Cult. 109, 439–445. 10.1007/s11240-011-0108-3 - DOI
LinkOut - more resources
Full Text Sources

