(Phenylamino)pyrimidine-1,2,3-triazole derivatives as analogs of imatinib: searching for novel compounds against chronic myeloid leukemia
- PMID: 34621389
- PMCID: PMC8450943
- DOI: 10.3762/bjoc.17.144
(Phenylamino)pyrimidine-1,2,3-triazole derivatives as analogs of imatinib: searching for novel compounds against chronic myeloid leukemia
Abstract
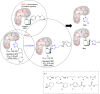
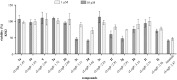
The enzyme tyrosine kinase BCR-Abl-1 is the main molecular target in the treatment of chronic myeloid leukemia and can be competitively inhibited by tyrosine kinase inhibitors such as imatinib. New potential competitive inhibitors were synthesized using the (phenylamino)pyrimidine-pyridine (PAPP) group as a pharmacophoric fragment, and these compounds were biologically evaluated. The synthesis of twelve new compounds was performed in three steps and assisted by microwave irradiation in a 1,3-dipolar cycloaddition to obtain 1,2,3-triazole derivatives substituted on carbon C-4 of the triazole nucleus. All compounds were evaluated for their inhibitory activities against a chronic myeloid leukemia cell line (K562) that expresses the enzyme tyrosine kinase BCR-Abl-1 and against healthy cells (WSS-1) to observe their selectivity. Three compounds showed promising results, with IC50 values between 1.0 and 7.3 μM, and were subjected to molecular docking studies. The results suggest that such compounds can interact at the same binding site as imatinib, probably sharing a competitive inhibition mechanism. One compound showed the greatest interaction affinity for BCR-Abl-1 in the docking studies.
Keywords: (phenylamino)pyrimidine-pyridine; 1,2,3-triazole; 1,3-dipolar cycloaddition; chronic myeloid leukemia; imatinib.
Copyright © 2021, Pimentel et al.
Figures



References
-
- de Azevedo L D, Bastos M M, de Oliveira A P, Boechat N. Quim Nova. 2017;40:791–809. doi: 10.21577/0100-4042.20170027. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
