Mesenchymal Stem Cell-Derived Exosomes and Their Potential Agents in Hematological Diseases
- PMID: 34621464
- PMCID: PMC8492257
- DOI: 10.1155/2021/4539453
Mesenchymal Stem Cell-Derived Exosomes and Their Potential Agents in Hematological Diseases
Retraction in
-
Retracted: Mesenchymal Stem Cell-Derived Exosomes and Their Potential Agents in Hematological Diseases.Oxid Med Cell Longev. 2024 Jan 9;2024:9869257. doi: 10.1155/2024/9869257. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 38234538 Free PMC article.
Abstract
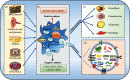
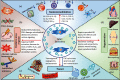
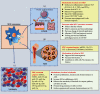
Mesenchymal stem cells (MSCs) are the most exploited stem cells with multilineage differentiation potential and immunomodulatory properties. Numerous lines of findings have reported their successful applications in a multitude of inflammatory conditions and immune disorders. However, it is currently discovered that these effects are mainly mediated in a paracrine manner by MSC-exosomes. Moreover, MSC-exosomes have been implicated in a wide variety of biological responses including immunomodulation, oxidative stress, tumor progression, and tissue regeneration. Meanwhile, they are reported to actively participate in various hematological diseases by the means of transferring different types of exosomal components to the target cells. Therefore, in this review, we briefly discuss the sources and biological features of MSCs and then illustrate the biogenesis and biological processes of MSC-exosomes. Of note, this paper especially highlights the latest research progress of MSC-exosomes in hematological diseases.
Copyright © 2021 Min Shen and Tong Chen.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

