Ezh2 harnesses the intranuclear actin cytoskeleton to remodel chromatin in differentiating Th cells
- PMID: 34622148
- PMCID: PMC8479699
- DOI: 10.1016/j.isci.2021.103093
Ezh2 harnesses the intranuclear actin cytoskeleton to remodel chromatin in differentiating Th cells
Abstract
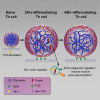
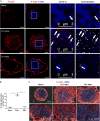
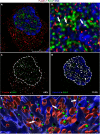
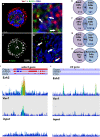
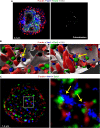
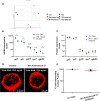
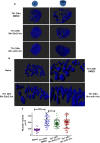
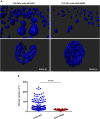
Following their first interaction with the antigen, quiescent naive T-helper (Th; CD4+) cells enlarge, differentiate, and proliferate; these processes are accompanied by substantial epigenetic alterations. We showed previously that the epigenetic regulators the polycomb-group (PcG) proteins have a dual function as both positive and negative transcriptional regulators; however, the underlying mechanisms remain poorly understood. Here, we demonstrate that during Th cell differentiation the methyltransferase activity of the PcG protein Ezh2 regulates post-transcriptionally inducible assembly of intranuclear actin filaments. These filaments are colocalized with the actin regulators Vav1 and WASp, vertically oriented to the T cell receptor, and intermingle with the chromatin fibers. Ezh2 and Vav1 are observed together at chromatin-actin intersections. Furthermore, the inducible assembly of nuclear actin filaments is required for chromatin spreading and nuclear growth. Altogether these findings delineate a model in which the epigenetic machinery orchestrates the dynamic mechanical force of the intranuclear cytoskeleton to reorganize chromatin during differentiation.
Keywords: Biological sciences; Cell biology; Immunology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ansel K.M., Djuretic I., Tanasa B., Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 2006;24:607–656. - PubMed
-
- Avni O., Koren O. Molecular (Me)micry? Cell Host Microbe. 2018;23:576–578. - PubMed
-
- Avni O., Lee D., Macian F., Szabo S.J., Glimcher L.H., Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat. Immunol. 2002;3:643–651. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

