Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer's-type dementia
- PMID: 34622235
- PMCID: PMC8484692
- DOI: 10.1016/j.xcrm.2021.100398
Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer's-type dementia
Abstract
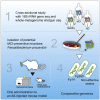
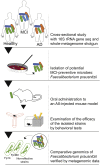
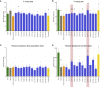
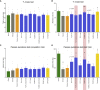
Evidence linking the gut-brain axis to Alzheimer's disease (AD) is accumulating, but the characteristics of causally important microbes are poorly understood. We perform a fecal microbiome analysis in healthy subjects and those with mild cognitive impairment (MCI) and AD. We find that Faecalibacterium prausnitzii (F. prausnitzii) correlates with cognitive scores and decreases in the MCI group compared with the healthy group. Two isolated strains from the healthy group, live Fp360 and pasteurized Fp14, improve cognitive impairment in an AD mouse model. Whole-genome comparison of isolated strains reveals specific orthologs that are found only in the effective strains and are more abundant in the healthy group compared with the MCI group. Metabolome and RNA sequencing analyses of mouse brains provides mechanistic insights into the relationship between the efficacy of pasteurized Fp14, oxidative stress, and mitochondrial function. We conclude that F. prausnitzii strains with these specific orthologs are candidates for gut microbiome-based intervention in Alzheimer's-type dementia.
Keywords: Alzheimer’s disease; Faecalibacterium prausnitzii; MCI; applied microbiology; comparative genomics; gut microbiome; gut microbiome-based intervention; metagenome; microbiota-gut-brain axis; mild cognitive impairment.
© 2021 The Author(s).
Conflict of interest statement
The Tokyo Institute of Technology and Otsuka Pharmaceutical Co., Ltd. are currently applying for a patent dealing with the use of F. prausnitzii and its components for prevention, diagnosis, and treatment of dementia. The inventors are A.K., S.S., T.Y., S.T., K.U., A.U., T. Kariya, T. Kawahara, Y.K., and N.K. T.Y. is a founder of Metabologenomics and Metagen Therapeutics. The companies focus on design and control of the gut environment for human health but had no control over the experiments, interpretations, writing, or publication of this work. The terms of these arrangements are being managed by the Tokyo Metropolitan Institute of Gerontology; Otsuka Pharmaceutical Co., Ltd.; and the Tokyo Institute of Technology in accordance with their conflict of interest policies.
Figures




References
-
- World Health Organization . 2017. Global action plan on the public health response to dementia 2017–2025.https://www.who.int/mental_health/neurology/dementia/action_plan_2017_20...
-
- Haeberlein S.B., von Hehn C., Tian Y., Chalkias S., Muralidharan K.K., Chen T., Wu S., Li J., Skordos L., Nisenbaum L. 2020. EMERGE and ENGAGE Topline Results: Two Phase 3 Studies to Evaluate Aducanumab in Patients With Early Alzheimer’s Disease. In: AAN Science Highlights.https://investors.biogen.com/static-files/ccb1c817-6d20-4987-b9dc-bcfa96...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

