T cell protein tyrosine phosphatase protects intestinal barrier function by restricting epithelial tight junction remodeling
- PMID: 34623320
- PMCID: PMC8409587
- DOI: 10.1172/JCI138230
T cell protein tyrosine phosphatase protects intestinal barrier function by restricting epithelial tight junction remodeling
Abstract
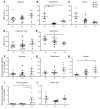
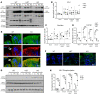
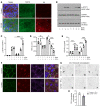
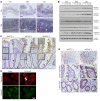
Genome-wide association studies revealed that loss-of-function mutations in protein tyrosine phosphatase non-receptor type 2 (PTPN2) increase the risk of developing chronic immune diseases, such as inflammatory bowel disease (IBD) and celiac disease. These conditions are associated with increased intestinal permeability as an early etiological event. The aim of this study was to examine the consequences of deficient activity of the PTPN2 gene product, T cell protein tyrosine phosphatase (TCPTP), on intestinal barrier function and tight junction organization in vivo and in vitro. Here, we demonstrate that TCPTP protected against intestinal barrier dysfunction induced by the inflammatory cytokine IFN-γ by 2 mechanisms: it maintained localization of zonula occludens 1 and occludin at apical tight junctions and restricted both expression and insertion of the cation pore-forming transmembrane protein, claudin-2, at tight junctions through upregulation of the inhibitory cysteine protease, matriptase. We also confirmed that the loss-of-function PTPN2 rs1893217 SNP was associated with increased intestinal claudin-2 expression in patients with IBD. Moreover, elevated claudin-2 levels and paracellular electrolyte flux in TCPTP-deficient intestinal epithelial cells were normalized by recombinant matriptase. Our findings uncover distinct and critical roles for epithelial TCPTP in preserving intestinal barrier integrity, thereby proposing a mechanism by which PTPN2 mutations contribute to IBD.
Keywords: Gastroenterology; Inflammation; Inflammatory bowel disease; Phosphoprotein phosphatases; Tight junctions.
Conflict of interest statement
Figures



Comment in
-
PTPN2 mutations cause epithelium-intrinsic barrier loss that synergizes with mucosal immune hyperactivation.J Clin Invest. 2021 Sep 1;131(17):e151414. doi: 10.1172/JCI151414. J Clin Invest. 2021. PMID: 34623321 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

