Time Taken to Detect and Respond to Polio Outbreaks in Africa and the Potential Impact of Direct Molecular Detection and Nanopore Sequencing
- PMID: 34623444
- PMCID: PMC9417130
- DOI: 10.1093/infdis/jiab518
Time Taken to Detect and Respond to Polio Outbreaks in Africa and the Potential Impact of Direct Molecular Detection and Nanopore Sequencing
Abstract
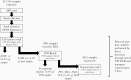
Background: Detection of poliovirus outbreaks relies on a complex laboratory algorithm of cell-culture, polymerase chain reaction (PCR), and sequencing to distinguish wild-type and vaccine-derived polioviruses (VDPV) from Sabin-like strains. We investigated the potential for direct molecular detection and nanopore sequencing (DDNS) to accelerate poliovirus detection.
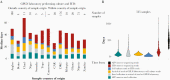
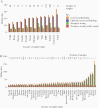
Methods: We analyzed laboratory data for time required to analyze and sequence serotype-2 VDPV (VDPV2) in stool collected from children with acute flaccid paralysis in Africa (May 2016-February 2020). Impact of delayed detection on VDPV2 outbreak size was assessed through negative binomial regression.
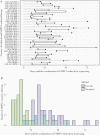
Results: VDPV2 confirmation in 525 stools required a median of 49 days from paralysis onset (10th-90th percentile, 29-74), comprising collection and transport (median, 16 days), cell-culture (7 days), intratypic differentiation quantitative reverse transcription PCR (3 days), and sequencing, including shipping if required (15 days). New VDPV2 outbreaks were confirmed a median of 35 days (27-60) after paralysis onset, which we estimate could be reduced to 16 days by DDNS (9-37). Because longer delays in confirmation and response were positively associated with more cases (P < .001), we estimate that DDNS could reduce the number of VDPV2 cases before a response by 28% (95% credible interval, 12%-42%).
Conclusions: DDNS could accelerate poliovirus outbreak response, reducing their size and the cost of eradication.
Keywords: AFP; VDPV; direct detection; nanopore; outbreak; poliovirus; stool; surveillance.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

