Riboswitch-mediated inducible expression of an astaxanthin biosynthetic operon in plastids
- PMID: 34623449
- PMCID: PMC8774745
- DOI: 10.1093/plphys/kiab428
Riboswitch-mediated inducible expression of an astaxanthin biosynthetic operon in plastids
Abstract
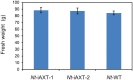
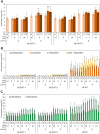
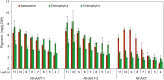
The high-value carotenoid astaxanthin (3,3'-dihydroxy-β,β-carotene-4,4'-dione) is one of the most potent antioxidants in nature. In addition to its large-scale use in fish farming, the pigment has applications as a food supplement and an active ingredient in cosmetics and in pharmaceuticals for the treatment of diseases linked to reactive oxygen species. The biochemical pathway for astaxanthin synthesis has been introduced into seed plants, which do not naturally synthesize this pigment, by nuclear and plastid engineering. The highest accumulation rates have been achieved in transplastomic plants, but massive production of astaxanthin has resulted in severe growth retardation. What limits astaxanthin accumulation levels and what causes the mutant phenotype is unknown. Here, we addressed these questions by making astaxanthin synthesis in tobacco (Nicotiana tabacum) plastids inducible by a synthetic riboswitch. We show that, already in the uninduced state, astaxanthin accumulates to similarly high levels as in transplastomic plants expressing the pathway constitutively. Importantly, the inducible plants displayed wild-type-like growth properties and riboswitch induction resulted in a further increase in astaxanthin accumulation. Our data suggest that the mutant phenotype associated with constitutive astaxanthin synthesis is due to massive metabolite turnover, and indicate that astaxanthin accumulation is limited by the sequestration capacity of the plastid.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures





References
-
- Aider A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

