Ketamine and other glutamate receptor modulators for depression in adults with bipolar disorder
- PMID: 34623633
- PMCID: PMC8499740
- DOI: 10.1002/14651858.CD011611.pub3
Ketamine and other glutamate receptor modulators for depression in adults with bipolar disorder
Abstract
Background: Glutamergic system dysfunction has been implicated in the pathophysiology of bipolar depression. This is an update of the 2015 Cochrane Review for the use of glutamate receptor modulators for depression in bipolar disorder.
Objectives: 1. To assess the effects of ketamine and other glutamate receptor modulators in alleviating the acute symptoms of depression in people with bipolar disorder. 2. To review the acceptability of ketamine and other glutamate receptor modulators in people with bipolar disorder who are experiencing depressive symptoms.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Embase and PsycINFO all years to July 2020. We did not apply any restrictions to date, language or publication status.
Selection criteria: RCTs comparing ketamine or other glutamate receptor modulators with other active psychotropic drugs or saline placebo in adults with bipolar depression.
Data collection and analysis: Two review authors independently selected studies for inclusion, assessed trial quality and extracted data. Primary outcomes were response rate and adverse events. Secondary outcomes included remission rate, depression severity change scores, suicidality, cognition, quality of life, and dropout rate. The GRADE framework was used to assess the certainty of the evidence.
Main results: Ten studies (647 participants) were included in this review (an additional five studies compared to the 2015 review). There were no additional studies added to the comparisons identified in the 2015 Cochrane review on ketamine, memantine and cytidine versus placebo. However, three new comparisons were found: ketamine versus midazolam, N-acetylcysteine versus placebo, and riluzole versus placebo. The glutamate receptor modulators studied were ketamine (three trials), memantine (two), cytidine (one), N-acetylcysteine (three), and riluzole (one). Eight of these studies were placebo-controlled and two-armed. In seven trials the glutamate receptor modulators had been used as add-on drugs to mood stabilisers. Only one trial compared ketamine with an active comparator, midazolam. The treatment period ranged from a single intravenous administration (all ketamine studies), to repeated administration for riluzole, memantine, cytidine, and N-acetylcysteine (with a follow-up of eight weeks, 8 to 12 weeks, 12 weeks, and 16 to 20 weeks, respectively). Six of the studies included sites in the USA, one in Taiwan, one in Denmark, one in Australia, and in one study the location was unclear. All participants had a primary diagnosis of bipolar disorder and were experiencing an acute bipolar depressive episode, diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders fourth edition (IV) or fourth edition text revision (IV-TR). Among all glutamate receptor modulators included in this review, only ketamine appeared to be more efficacious than placebo 24 hours after infusion for response rate (odds ratio (OR) 11.61, 95% confidence interval (CI) 1.25 to 107.74; P = 0.03; participants = 33; studies = 2; I² = 0%, low-certainty evidence). Ketamine seemed to be more effective in reducing depression rating scale scores (MD -11.81, 95% CI -20.01 to -3.61; P = 0.005; participants = 32; studies = 2; I2 = 0%, very low-certainty evidence). There was no evidence of ketamine's efficacy in producing remission over placebo at 24 hours (OR 5.16, 95% CI 0.51 to 52.30; P = 0.72; participants = 33; studies = 2; I2 = 0%, very low-certainty evidence). Evidence on response, remission or depression rating scale scores between ketamine and midazolam was uncertain at 24 hours due to very low-certainty evidence (OR 3.20, 95% CI 0.23 to 45.19). In the one trial assessing ketamine and midazolam, there were no dropouts due to adverse effects or for any reason (very low-certainty evidence). Placebo may have been more effective than N-acetylcysteine in reducing depression rating scale scores at three months, although this was based on very low-certainty evidence (MD 1.28, 95% CI 0.24 to 2.31; participants = 58; studies = 2). Very uncertain evidence found no difference in response at three months (OR 0.82, 95% CI 0.32 to 2.14; participants = 69; studies = 2; very low-certainty evidence). No data were available for remission or acceptability. Extremely limited data were available for riluzole vs placebo, finding only very-low certainty evidence of no difference in dropout rates (OR 2.00, 95% CI 0.31 to 12.84; P = 0.46; participants = 19; studies = 1; I2 = 0%).
Authors' conclusions: It is difficult to draw reliable conclusions from this review due to the certainty of the evidence being low to very low, and the relatively small amount of data usable for analysis in bipolar disorder, which is considerably less than the information available for unipolar depression. Nevertheless, we found uncertain evidence in favour of a single intravenous dose of ketamine (as add-on therapy to mood stabilisers) over placebo in terms of response rate up to 24 hours, however ketamine did not show any better efficacy for remission in bipolar depression. Even though ketamine has the potential to have a rapid and transient antidepressant effect, the efficacy of a single intravenous dose may be limited. We did not find conclusive evidence on adverse events with ketamine, and there was insufficient evidence to draw meaningful conclusions for the remaining glutamate receptor modulators. However, ketamine's psychotomimetic effects (such as delusions or delirium) may have compromised study blinding in some studies, and so we cannot rule out the potential bias introduced by inadequate blinding procedures. To draw more robust conclusions, further methodologically sound RCTs (with adequate blinding) are needed to explore different modes of administration of ketamine, and to study different methods of sustaining antidepressant response, such as repeated administrations.
Trial registration: ClinicalTrials.gov NCT01797575 NCT00088699 NCT00088699 NCT01881763.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
RD, TM, CH, SS, AB, RS, PJC, KH and JG report no competing interests.
RM runs NHS and self‐pay ketamine clinics for Oxford Health NHS Foundation Trust. RM has undertaken educational and scientific advisory board work for Janssen Pharmaceuticals to support educational and research activity, no funds are received personally. Janssen supported RM's attendance at the APA conference in New York in 2018. RM has undertaken scientific advisory board work for Sage pharmaceuticals, no funds are directly received. RM is supported by the NIHR Oxford Health Biomedical Research Centre.
GSM has received grant or research support from National Health and Medical Research Council, Australian Rotary Health, NSW Health, American Foundation for Suicide Prevention, Ramsay Research and Teaching Fund, Elsevier, AstraZeneca, Janssen‐Cilag, Lundbeck, Otsuka and Servier; and has been a consultant for AstraZeneca, Janssen‐Cilag, Lundbeck, Otsuka and Servier.
AC has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation and Angelini Pharma.
Figures
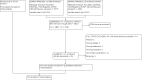
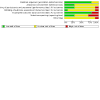
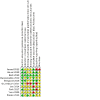































Update of
-
Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults.Cochrane Database Syst Rev. 2015 Sep 29;(9):CD011611. doi: 10.1002/14651858.CD011611.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 Oct 8;10:CD011611. doi: 10.1002/14651858.CD011611.pub3. PMID: 26415966 Updated.
References
References to studies included in this review
Anand 2012 {published data only}
-
- Anand A, Gunn AD, Barkay G, Karne HS, Nurnberger JI, Mathew SJ, et al. Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disorders 2012;14:64-70. - PubMed
Bauer 2019 {published and unpublished data}
-
- Bauer I, Green C, Coplo GD, Teixeira AL, Selvaraj, Durkin K, et al. A double-blind, randomized, placebo-controlled study of aspirin and N-Acetylcysteine as adjunctive treatments for bipolar depression. Journal of Clinical Psychiatry 2019;80(1):18m12200. - PubMed
Berk 2019 {published data only}
Diazgranados 2010 {published and unpublished data}
Ellegaard 2019 {published data only}
-
- Ellegard PK, Licht RW, Nielson RE, Dean OM, Berk M, Poulsen HE, et al. The efficacy of adjunctive N-acetylcysteine in acute bipolar depression: a randomized placebo-controlled study. Journal of Affective Disorders 2019;245:1043-51. - PubMed
Grunebaum 2017 {published and unpublished data}
Lee 2014 {published data only}
-
- Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, et al. The effects of add-on low-dose memantine on cytokine levels in bipolar II depression: a 12-week double-blind, randomized controlled trial. Journal of Clinical Psychopharmacology 2014;34:337-43. - PubMed
Park 2017 {published data only}
Yoon 2009 {published data only}
-
- Yoon SJ, Lyoo IK, Haws C, Kim TS, Cohen BM, Renshaw PF. Decreased glutamate/glutamine levels may mediate cytidine's efficacy in treating bipolar depression: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology 2009;34(7):1810-8. - PubMed
Zarate 2012 {published and unpublished data}
References to studies excluded from this review
Alda 2017 {published data only}
-
- Alda M, McKinnon M, Blagdon R, Garnham J, MacLellan S, O'donovan C, et al. Methylene blue treatment for residual symptoms of bipolar disorder: randomised crossover study. British Journal of Psychiatry 2017;210(1):54-60. - PubMed
Berk 2008 {published data only}
-
- Magalhães PV, Dean OM, Bush AI, Copolov DL, Weisinger D, Malhi GS, et al. Systemic illness moderates the impact of N-acetyl cysteine in bipolar disorder. Progress in Neuropsychopharmacology and Biological Psychiatry 2012;37(1):132-5. - PubMed
-
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder-a double-blind randomized placebo-controlled trial. Biological Psychiatry 2008;64(6):468-75. - PubMed
-
- Dean O, Berk M, Cotton SM, Bush AI, Gama CS, Kapczinski F, et al. N-acetyl cysteine (NAC) as an adjunctive therapy for bipolar depression conference poster P35. In: Bipolar disorders. Abstracts of the 9th International Conference on Bipolar Disorder; 2011 June 9-11. Vol. 13. Pittsburgh [PA], 2011:38.
Berk 2010 {published data only}
-
- Berk M, Dodd S, Dean OM, Kohlmann K, Berk L, Malhi GS. The validity and internal structure of the Bipolar Depression Rating Scale: data from a clinical trial of N-acetylcysteine as adjunctive therapy in bipolar disorder. Acta Neuropsychiatrica 2010;22(5):237-42. - PubMed
Castillo 2017 {published data only}
-
- Castillo MF, Murata S, Schwarz M, Martin B, Halaris A. Celecoxib augmentation of escitalopram in treatment-resistant bipolar depression: effects on trycats. Neuropsychopharmacology 2017;43:S176-7.
Chen 2014 {published data only}
-
- Chen SL, Lee SY, Chang YH, Chen PS, Wang TY, Lu RB. Therapeutic effects of add-on low-dose dextromethorphan plus valproic acid in bipolar disorder. European Neuropsychopharmacology 2014;24(11):1753-9. - PubMed
Cocchi 1977 {published data only}
-
- Cocchi R, Fusari A, Lorini G, Roccia L. Acupunctura, "vital" drugs and psychopharmacological agents in the treatment of psychiatric patients with deep depressive disorders, with considerations on the probable neurophysiological mechanisms of the synergism of action [article in Italian]. Minerva Medica 1977;68(33):2309-14. - PubMed
Ehrensing 1978 {published data only}
-
- Ehrensing RH, Kastin AJ. Dose-related biphasic effect of prolyl-leucyl-glycinamide (MIF-I) in depression. American Journal of Psychiatry 1978;135(5):562-6. - PubMed
Ellis 2014 {published data only}
-
- Ellis JS, Luckenbaugh DA, Zarate CA, Furey ML. Effects of ketamine versus scopolamine on individual depression and anxiety symptoms (abstract). Biological Psychiatry 2014;75:127S.
Kantrowitz 2015 {published data only}
-
- Kantrowitz JT, Halberstam B, Gangwisch J. Single-dose ketamine followed by daily D-Cycloserine in treatment-resistant bipolar depression. Journal of Clinical Psychiatry 2015;76(6):737-8. - PubMed
Lee 2012 {published data only}
-
- Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, et al. The DRD2/ANKK1 gene is associated with response to add-on dextromethorphan treatment in bipolar disorder. Journal of Affective Disorders 2012;138:295-300. - PubMed
Lee 2017 {published data only}
-
- Lee SY, Chen SL, Wang TY, Chang YH, Chen PS, Huang SY, et al. The COMT Val158Met Polymorphism is associated with response to add-on dextromethorphan treatment in bipolar disorder. Journal of Clinical Pharmacology 2017;37(1):94-8. - PubMed
References to ongoing studies
ACTRN12612000830897 {published data only}
-
- ACTRN12612000830897. A double blind, placebo controlled, randomised trial to evaluate the effect of mitochondrial agents, N-acetyl cysteine or placebo on the depressive phase of bipolar disorder. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000830897 (first received 23 July 2012).
ISRCTN14689382 {published data only}14689382
-
- ISRCTN14689382. Ketamine augmentation of ECT to improve outcomes in depression. www.isrctn.org/ISRCTN14689382.
NCT01881763 {published data only}
-
- NCT01881763. Comparing therapeutic efficacy and cognitive side effects of electroconvulsive therapy (ECT) using ketamine versus methohexital anesthesia. www.clinicaltrials.gov/show/NCT01881763 (first received 20 June 2013).
NCT03396068 {unpublished data only}
-
- NCT03396068. NRX-101 for maintenance of remission from severe bipolar depression in patients with suicidal ideation. ClinicalTrials.gov/show/NCT03396068 (first received 10 January 2018).
NCT03396601 {unpublished data only}
-
- NCT03396601. NRX100 vs. placebo for rapid stabilization of acute suicidal ideation and behavior in bipolar depression (SevereBD). ClinicalTrials.gov/show/NCT03396601 (first received 11 January 2018).
Additional references
Abdallah 2018
-
- Abdallah C, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double-blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv 2018. [DOI: doi.org/10.1101/500959 ]
Alberich 2017
Altman 1996
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edition. Washington D.C.: American Psychiatric Association, 1980.
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edition. Washington D.C.: American Psychiatric Association, 1987.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, D.C.: American Psychiatric Association, 1994.
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders - Text Revision (DSM-IV-TR). 4th edition. Washington, D.C.: American Psychiatric Association, 2000.
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington (VA): American Psychiatric Association, 2013.
Arroll 2009
Atkins 2004
Bandelow 2006
-
- Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder? Journal of Clinical Psychiatry 2006;67(9):1428–34. - PubMed
Bauer 2014
Berton 2006
-
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature Reviews. Neuroscience 2006;7(2):137–51. - PubMed
Bonaventura 2021
Brookes 2001
-
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomized controlled trials: quantifying the risks of false-positives and false-negatives. Health Technology Assessment 2001;5(33):1–56. - PubMed
Brookes 2004
-
- Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. Journal of Clinical Epidemiology 2004;57(3):229-36. - PubMed
Caddy 2015
Chitty 2013
Cipriani 2009
-
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009;373(9665):746–58. - PubMed
Cipriani 2010
Cipriani 2012
Cipriani 2013a
-
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 2013;346:f3646. - PubMed
Cipriani 2013b
Cipriani 2020
Cohen 2019
-
- Coehn, BM. Evidence-based drug treatment of acute depression in bipolar disorder. JAMA Psychiatry 2019;76(12):1314-5. - PubMed
Coyle 2015
-
- Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta‐analysis. Human Psychopharmacology: Clinical and Experimental 2015;30(3):152-63. - PubMed
Cunningham 2000
Dean 2021
-
- Dean RL, Hurducas C, Hawton K, Spyridi S, Cowen PJ, Hollingsworth S, et al. Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder. Cochrane Database of Systematic Reviews 2021, Issue 9. Art. No: CD011612. [DOI: 10.1002/14651858.CD011612.pub3] - DOI - PMC - PubMed
Dozois 2004
-
- Dozois DJ, Dobson KS. Depression. In: Antony MM, Barlow DH, editors(s). Handbook of Assessment and Treatment Planning for Psychological Disorders. New York: Guilford Press, 2004:259-99.
Efthimiou 2019
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving crossover trials: methodological issues. International Journal of Epidemiology 2002;31(1):140–9. - PubMed
Feighner 1972
-
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26(1):57–63. - PubMed
Furukawa 2002
-
- Furukawa TA, Guyatt GH, Griffith LE. An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002;31(1):72–6. - PubMed
Furukawa 2005
-
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta-analysis. International Clinical Psychopharmacology 2005;20(1):49–52. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7–10. - PubMed
Furukawa 2007a
-
- Furukawa TA, Watanabe N, Omori IM, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta-analyses. JAMA 2007;297(5):468–70. - PubMed
Furukawa 2007b
-
- Furukawa TA, Akechi T, Azuma H, Okuyama T, Higuchi T. Evidence-based guidelines for interpretation of the Hamilton Rating Scale for Depression. Journal of Clinical Psychopharmacology 2007;27(5):531–4. - PubMed
Geddes 2013
Gigante 2012
-
- Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disorders 2012;14:478-87. [DOI: doi: 10.1111/j.1399-5618.2012.01033.x] - PubMed
Godlewska 2019
Goodwin 2016
-
- Goodwin GM, Haddad PM, Ferrier IN, Aronson JK, Barnes T, Cipriani A, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology 2016;30(6):495-553. [DOI: 10.1177/0269881116636545] - DOI - PMC - PubMed
GRADEproGDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.) GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Version 2015. Hamilton: McMaster University (developed by Evidence Prime, Inc.), August 28th 2015.
Grady 2017
Guaiana 2010
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): U.S. Department of Health and Human Services, 1976.
Guyatt 1998
Hamilton 1960
Hidalgo‐Mazzei 2019
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane–handbook.org.
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane–handbook.org.
Higgins 2011d
-
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane–handbook.org.
Honey 2003
Joseph 2021
Judd 2002
-
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Archives of General Psychiatry 2002;59(6):530-7. - PubMed
Judd 2003
-
- Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Archives of General Psychiatry 2003;60(3):261-9. - PubMed
Kendall 2014
-
- Kendall T, Morriss R, Mayo-Wilson E, Marcus E, Guideline Development Group. Assessment and management of bipolar disorder: summary of updated NICE guidance. BMJ 2014;349:g5673. - PubMed
Kraus 2017
-
- Kraus C, Rabl U, Vanicek T, Carlberg L Popovic A. Administration of ketamine for unipolar and bipolar depression. International Journal of Psychiatry in Clinical Practice 2017;21(1):2-12. - PubMed
Kryst 2020
Kubitz 2013
Li 2010
Li 2018
Magni 2013
Malhi 2016
Malhi 2018
-
- Malhi GS, Outhred T, Das P, Morris G, Hamilton A, Mannie Z. Modeling suicide in bipolar disorders. Bipolar Disorders 2018;20(4):334-48. - PubMed
Malhi 2020a
Malhi 2020b
Malhi 2020c
Malhi 2021
McIntyre 2020
-
- McIntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, Kessing LV, et al. Bipolar Disorders. Lancet 2020;396(10265):1841-56. - PubMed
McMullen 2021
Merikangas 2011
Mitchell 2011
-
- Mitchell PB, Frankland A, Hadzi-Pavlovic D, Roberts G, Corry J, Wright A, et al. Comparison of depressive episodes in bipolar disorder and in major depressive disorder within bipolar disorder pedigrees. British Journal of Psychiatry 2011;199(4):303-9. - PubMed
Moher 2009
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382–9. - PubMed
Morgan 2010
-
- Morgan CJA, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction 2010;105:121-33. - PubMed
Moriguchi 2019
Muller 2003
-
- Muller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). Journal of Affective Disorders 2003;77(3):255–60. - PubMed
Murray 2014
Naughton 2014
-
- Naughton M, Clarke G, O'Leary OF, Cryan JF, Dinan TG. A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. Journal of Affective Disorders 2014;156:24-35. - PubMed
Niciu 2014
Phelps 2009
Pompili 2013
Purgato 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salvadore 2010
Schwartz 2016
Smith 2018
Spitzer 1978
-
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35(6):773–82. - PubMed
Stanislaus 2020
-
- Stanislaus S, Vinberg M, Melbye S, Frost M, Busk J, Bardram JE, et al. Daily self-reported and automatically generated smartphone-based sleep measurements in patients with newly diagnosed bipolar disorder, unaffected first-degree relatives and healthy control individuals. Evidence-Based Mental Health 2020;23(4):146-53. [DOI: 10.1136/ebmental-2020-300148] - DOI - PMC - PubMed
Sterne 2000
-
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119–29. - PubMed
Suh 1997
-
- Suh T, Gallo JJ. Symptom profiles of depression among general medical service users compared with specialty mental health service users. Psychological Medicine 1997;27(5):1051–63. - PubMed
Taylor 2014
-
- Taylor DM, Cornelius V, Smith L, Young AH. Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple-treatments meta-analysis. Acta Psychiatrica Scandinavica 2014;130(6):452-69. - PubMed
Thomas 2010
-
- Thomas SP, Nandhra HS, Jarvaraman A. Systematic review of lamotrigine augmentation of treatment resistant unipolar depression (TRD). Journal of Mental Health 2010;19(2):168-75. - PubMed
Wang 2015
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36-item short form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473–83. - PubMed
Ware 1998
-
- Ware JE, Kosinski M, Keller SD. SF-12: How to Score the SF-12. Physical and Mental Health Summary Scales. Lincoln (RI): QualityMetric Inc., 1998.
WHO 1992
-
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders. Geneva: WHO, 1992.
WHO 2018
-
- World Health Organization (WHO). International statistical classification of diseases and related health problems (11th Revision). Retrieved from https://icd.who.int/browse11/l-m/en.
WHOQOL Group 1998
-
- WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social Science and Medicine 1998;46(12):1569–85. - PubMed
Wilkinson 2019
Williams 2018
Wing 1998
-
- Wing JK, Beevor AS, Curtis RH, Park SB, Burns A. Health of the nation outcome scales (HoNOS). Research and development. British Journal of Psychiatry 1998;172:11-8. - PubMed
Witt 2020
-
- Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, Cipriani A, et al. Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials.. Australian and New Zealand JournL of Psychiatry 2020;54(1):29-45. [DOI: <html><body id="body">doi: 10.1177/0004867419883341</body></html>] - PubMed
Zarate 2006
-
- Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. American Journal of Psychiatry 2006;163(1):153-5.. - PubMed
Zavodnick 2012
-
- Zavodnick AD, Ali R. Lamotrigine in the treatment of unipolar depression with and without comorbidities: a literature review. Psychiatric Quarterly 2012;83:371-83. - PubMed

