Prolactin-sensitive olfactory sensory neurons regulate male preference in female mice by modulating responses to chemosensory cues
- PMID: 34623921
- PMCID: PMC8500514
- DOI: 10.1126/sciadv.abg4074
Prolactin-sensitive olfactory sensory neurons regulate male preference in female mice by modulating responses to chemosensory cues
Abstract
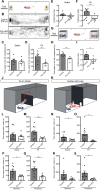
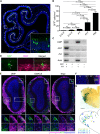
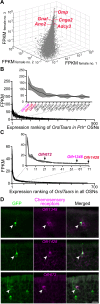
Chemosensory cues detected in the nose need to be integrated with the hormonal status to trigger appropriate behaviors, but the neural circuits linking the olfactory and the endocrine system are insufficiently understood. Here, we characterize olfactory sensory neurons in the murine nose that respond to the pituitary hormone prolactin. Deletion of prolactin receptor in these cells results in impaired detection of social odors and blunts male preference in females. The prolactin-responsive olfactory sensory neurons exhibit a distinctive projection pattern to the brain that is similar across different individuals and express a limited subset of chemosensory receptors. Prolactin modulates the responses within these neurons to discrete chemosensory cues contained in male urine, providing a mechanism by which the hormonal status can be directly linked with distinct olfactory cues to generate appropriate behavioral responses.
Figures


References
-
- Halpern M., Martinez-Marcos A., Structure and function of the vomeronasal system: An update. Prog. Neurobiol. 70, 245–318 (2003). - PubMed
-
- Touhara K., Vosshall L. B., Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 71, 307–332 (2009). - PubMed
-
- Baum M. J., Cherry J. A., Processing by the main olfactory system of chemosignals that facilitate mammalian reproduction. Horm. Behav. 68, 53–64 (2015). - PubMed
-
- Buck L., Axel R., A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65, 175–187 (1991). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

