Corticotropin releasing factor and norepinephrine related circuitry changes in the bed nucleus of the stria terminalis in stress and alcohol and substance use disorders
- PMID: 34624301
- PMCID: PMC8578398
- DOI: 10.1016/j.neuropharm.2021.108814
Corticotropin releasing factor and norepinephrine related circuitry changes in the bed nucleus of the stria terminalis in stress and alcohol and substance use disorders
Abstract
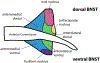
Alcohol Use Disorder (AUD) affects around 14.5 million individuals in the United States, with Substance Use Disorder (SUD) affecting an additional 8.3 million individuals. Relapse is a major barrier to effective long-term treatment of this illness with stress often described as a key trigger for a person with AUD or SUD to relapse during a period of abstinence. Two signaling molecules, norepinephrine (NE) and corticotropin releasing factor (CRF), are released during the stress response, and also play important roles in reward behaviors and the addiction process. Within the addiction literature, one brain region in which there has been increasing research focus in recent years is the bed nucleus of the stria terminalis (BNST). The BNST is a limbic structure with numerous cytoarchitecturally and functionally different subregions that has been implicated in drug-seeking behaviors and stress responses. This review focuses on drug and stress-related neurocircuitry changes in the BNST, particularly within the CRF and NE systems, with an emphasis on differences and similarities between the major dorsal and ventral BNST subregions.
Keywords: AUD; Adrenergic receptor; Alcohol; Alcohol use disorder; BNST; CRF; Corticotropin releasing factor; Drug use; Norepinephrine; Stress; Substance use disorder.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
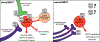
References
-
- Albrechet-Souza L, Viola TW, Grassi-Oliveira R, Miczek KA, de Almeida RMM, 2017. Corticotropin Releasing Factor in the Bed Nucleus of the Stria Terminalis in Socially Defeated and Non-stressed Mice with a History of Chronic Alcohol Intake. Front. Pharmacol 8, 762. 10.3389/fphar.2017.00762 - DOI - PMC - PubMed
-
- Bale TL, Vale WW, 2004. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol 44, 525. - PubMed

