The interactions of molecular chaperones with client proteins: why are they so weak?
- PMID: 34624315
- PMCID: PMC8567204
- DOI: 10.1016/j.jbc.2021.101282
The interactions of molecular chaperones with client proteins: why are they so weak?
Abstract
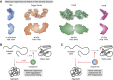
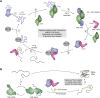
The major classes of molecular chaperones have highly variable sequences, sizes, and shapes, yet they all bind to unfolded proteins, limit their aggregation, and assist in their folding. Despite the central importance of this process to protein homeostasis, it has not been clear exactly how chaperones guide this process or whether the diverse families of chaperones use similar mechanisms. For the first time, recent advances in NMR spectroscopy have enabled detailed studies of how unfolded, "client" proteins interact with both ATP-dependent and ATP-independent classes of chaperones. Here, we review examples from four distinct chaperones, Spy, Trigger Factor, DnaK, and HscA-HscB, highlighting the similarities and differences between their mechanisms. One striking similarity is that the chaperones all bind weakly to their clients, such that the chaperone-client interactions are readily outcompeted by stronger, intra- and intermolecular contacts in the folded state. Thus, the relatively weak affinity of these interactions seems to provide directionality to the folding process. However, there are also key differences, especially in the details of how the chaperones release clients and how ATP cycling impacts that process. For example, Spy releases clients in a largely folded state, while clients seem to be unfolded upon release from Trigger Factor or DnaK. Together, these studies are beginning to uncover the similarities and differences in how chaperones use weak interactions to guide protein folding.
Keywords: chaperone; nuclear magnetic resonance (NMR); protein aggregation; protein folding; protein–protein interactions (PPIs).
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

