To control floating drug delivery system in a simulated gastric environment by adjusting the Shell layer formulation
- PMID: 34625115
- PMCID: PMC8501548
- DOI: 10.1186/s40824-021-00234-6
To control floating drug delivery system in a simulated gastric environment by adjusting the Shell layer formulation
Abstract
Background: Gastroretentive drug delivery system (GDDS) are novel systems that have been recently developed for treating stomach diseases. The key function of all GDDS systems is to control the retention time in the stomach. However, research into the bulk density or entanglement of polymers, especially regarding their effects on drug float and release times, is scarce.
Methods: In this research, we prepared the floating core-shell beads carrying tetracycline. The ratio of chitosan and xanthan gum in the shell layer was changed to modify polymer compactness. Tetracycline was encapsulated in the alginate core.
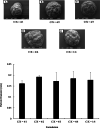
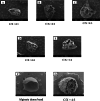
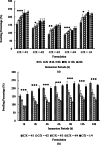
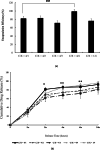
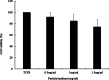
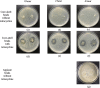
Results: Using scanning electron microscopy (SEM) techniques, we observed that the shell formulation did not change the bead morphology. The cross-sectional images showed that the beads were highly porous. The interaction between anionic xanthan gum and cationic chitosan made the shell layer dense, resisting to the mass transfer in the shell layer. Due to the high mass transfer resistance to water penetration, the longer float and delivery time were caused by the dense surface of the beads. The cell culture demonstrated that floating core-shell beads were biocompatible. Importantly, the beads with tetracycline showed a significant prolonged anti-bacterial effect.
Conclusion: Research results proved that the floating and releasing progress of core-shell beads can be well controlled by adjusting the shell layer formulation that could promote the function of gastroretentive drugs.
Keywords: Anti-bacterial effect; Chitosan; Core-shell particles; Floating beads; Gastroretentive drug delivery; Xanthan gum.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Patel SS, Ray S, Thakur RS. Formualtion and evaluation of floating drug delivery system containing clarithromycin for helicobacter pylori. Acta Pol Pharm. 2006;63:53–61. - PubMed
-
- Nayak RMAK, Biswarup D. Gastroretentive drug delivery systems: a review. Asian J Pharm Clin Res. 2010;3:2–10.
Grants and funding
LinkOut - more resources
Full Text Sources

