Circulating Tumor DNA in Stage III Colorectal Cancer, beyond Minimal Residual Disease Detection, toward Assessment of Adjuvant Therapy Efficacy and Clinical Behavior of Recurrences
- PMID: 34625408
- PMCID: PMC9401484
- DOI: 10.1158/1078-0432.CCR-21-2404
Circulating Tumor DNA in Stage III Colorectal Cancer, beyond Minimal Residual Disease Detection, toward Assessment of Adjuvant Therapy Efficacy and Clinical Behavior of Recurrences
Abstract
Purpose: Sensitive methods for risk stratification, monitoring therapeutic efficacy, and early relapse detection may have a major impact on treatment decisions and patient management for stage III colorectal cancer patients. Beyond assessing the predictive power of postoperative ctDNA detection, we explored the added benefits of serial analysis: assessing adjuvant chemotherapy (ACT) efficacy, early relapse detection, and ctDNA growth rates.
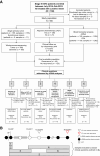
Experimental design: We recruited 168 patients with stage III colorectal cancer treated with curative intent at Danish and Spanish hospitals between 2014 and 2019. To quantify ctDNA in plasma samples (n = 1,204), 16 patient-specific somatic single-nucleotide variants were profiled using multiplex-PCR, next-generation sequencing.
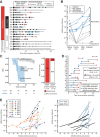
Results: Detection of ctDNA was a strong recurrence predictor postoperatively [HR = 7.0; 95% confidence interval (CI), 3.7-13.5; P < 0.001] and directly after ACT (HR = 50.76; 95% CI, 15.4-167; P < 0.001). The recurrence rate of postoperative ctDNA-positive patients treated with ACT was 80% (16/20). Only patients who cleared ctDNA permanently during ACT did not relapse. Serial ctDNA assessment after the end of treatment was similarly predictive of recurrence (HR = 50.80; 95% CI, 14.9-172; P < 0.001), and revealed two distinct rates of exponential ctDNA growth, slow (25% ctDNA-increase/month) and fast (143% ctDNA-increase/month; P < 0.001). The ctDNA growth rate was prognostic of survival (HR = 2.7; 95% CI, 1.1-6.7; P = 0.039). Serial ctDNA analysis every 3 months detected recurrence with a median lead-time of 9.8 months compared with standard-of-care computed tomography.
Conclusions: Serial postoperative ctDNA analysis has a strong prognostic value and enables tumor growth rate assessment. The novel combination of ctDNA detection and growth rate assessment provides unique opportunities for guiding decision-making.See related commentary by Morris and George, p. 438.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
Using Circulating Tumor DNA for Colon Cancer Adjuvant Therapy: To Be or Not to Be?Clin Cancer Res. 2022 Feb 1;28(3):438-440. doi: 10.1158/1078-0432.CCR-21-3564. Epub 2021 Nov 30. Clin Cancer Res. 2022. PMID: 34848532
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020;71:209–49. - PubMed
-
- Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up y on behalf of the ESMO Guidelines Committee. Ann Oncol 2020;31:1291–305. - PubMed
-
- Pahlman LA, Hohenberger WM, Matzel K, Sugihara K, Quirke P, Glimelius B. Should the benefit of adjuvant chemotherapy in colon cancer be re-evaluated? J Clin Oncol 2016;34:1297–9. - PubMed
-
- Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology. Dis Colon Rectum 2018;61:1016–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

