SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray
- PMID: 34625610
- PMCID: PMC8501059
- DOI: 10.1038/s41598-021-99404-8
SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray
Abstract
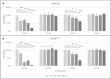
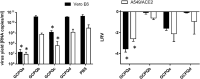
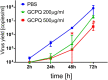
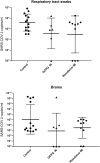
There are currently no cures for coronavirus infections, making the prevention of infections the only course open at the present time. The COVID-19 pandemic has been difficult to prevent, as the infection is spread by respiratory droplets and thus effective, scalable and safe preventive interventions are urgently needed. We hypothesise that preventing viral entry into mammalian nasal epithelial cells may be one way to limit the spread of COVID-19. Here we show that N-palmitoyl-N-monomethyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycolchitosan (GCPQ), a positively charged polymer that has been through an extensive Good Laboratory Practice toxicology screen, is able to reduce the infectivity of SARS-COV-2 in A549ACE2+ and Vero E6 cells with a log removal value of - 3 to - 4 at a concentration of 10-100 μg/ mL (p < 0.05 compared to untreated controls) and to limit infectivity in human airway epithelial cells at a concentration of 500 μg/ mL (p < 0.05 compared to untreated controls). In vivo studies using transgenic mice expressing the ACE-2 receptor, dosed nasally with SARS-COV-2 (426,000 TCID50/mL) showed a trend for nasal GCPQ (20 mg/kg) to inhibit viral load in the respiratory tract and brain, although the study was not powered to detect statistical significance. GCPQ's electrostatic binding to the virus, preventing viral entry into the host cells, is the most likely mechanism of viral inhibition. Radiolabelled GCPQ studies in mice show that at a dose of 10 mg/kg, GCPQ has a long residence time in mouse nares, with 13.1% of the injected dose identified from SPECT/CT in the nares, 24 h after nasal dosing. With a no observed adverse effect level of 18 mg/kg in rats, following a 28-day repeat dose study, clinical testing of this polymer, as a COVID-19 prophylactic is warranted.
© 2021. The Author(s).
Conflict of interest statement
IFU and AGS are directors of Nanomerics Ltd.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

