The evolving view of thermogenic adipocytes - ontogeny, niche and function
- PMID: 34625737
- PMCID: PMC8814904
- DOI: 10.1038/s41574-021-00562-6
The evolving view of thermogenic adipocytes - ontogeny, niche and function
Abstract
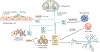
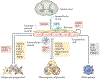
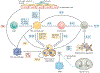
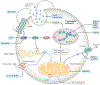
The worldwide incidence of obesity and its sequelae, such as type 2 diabetes mellitus, have reached pandemic levels. Central to the development of these metabolic disorders is adipose tissue. White adipose tissue stores excess energy, whereas brown adipose tissue (BAT) and beige (also known as brite) adipose tissue dissipate energy to generate heat in a process known as thermogenesis. Strategies that activate and expand BAT and beige adipose tissue increase energy expenditure in animal models and offer therapeutic promise to treat obesity. A better understanding of the molecular mechanisms underlying the development of BAT and beige adipose tissue and the activation of thermogenic function is the key to creating practical therapeutic interventions for obesity and metabolic disorders. In this Review, we discuss the regulation of the tissue microenvironment (the adipose niche) and inter-organ communication between BAT and other tissues. We also cover the activation of BAT and beige adipose tissue in response to physiological cues (such as cold exposure, exercise and diet). We highlight advances in harnessing the therapeutic potential of BAT and beige adipose tissue by genetic, pharmacological and cell-based approaches in obesity and metabolic disorders.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
Y.-H.T. is an inventor on US Patent 7,576,052 related to BMP7 and US patent applications related to 12,13-diHOME and FGF6/9. The other authors declare no competing interests.
Figures

Comment in
-
A mitochondrial trigger for beige adipocyte development.Nat Rev Endocrinol. 2023 Feb;19(2):65. doi: 10.1038/s41574-022-00792-2. Nat Rev Endocrinol. 2023. PMID: 36517630 No abstract available.
References
-
- Cannon B & Nedergaard J Brown adipose tissue: function and physiological significance. Physiol. Rev 84, 277–359 (2004). - PubMed
-
- Bartelt A et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med 17, 200–205 (2011). - PubMed
-
- Scheja L & Heeren J The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol 15, 507–524 (2019). - PubMed
-
- Villarroya F, Cereijo R, Villarroya J & Giralt M Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol 13, 26–35 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

