Combination of terbium-161 with somatostatin receptor antagonists-a potential paradigm shift for the treatment of neuroendocrine neoplasms
- PMID: 34625828
- PMCID: PMC8921065
- DOI: 10.1007/s00259-021-05564-0
Combination of terbium-161 with somatostatin receptor antagonists-a potential paradigm shift for the treatment of neuroendocrine neoplasms
Abstract
Purpose: The β¯-emitting terbium-161 also emits conversion and Auger electrons, which are believed to be effective in killing single cancer cells. Terbium-161 was applied with somatostatin receptor (SSTR) agonists that localize in the cytoplasm (DOTATOC) and cellular nucleus (DOTATOC-NLS) or with a SSTR antagonist that localizes at the cell membrane (DOTA-LM3). The aim was to identify the most favorable peptide/terbium-161 combination for the treatment of neuroendocrine neoplasms (NENs).
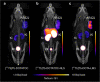
Methods: The capability of the 161Tb- and 177Lu-labeled somatostatin (SST) analogues to reduce viability and survival of SSTR-positive AR42J tumor cells was investigated in vitro. The radiopeptides' tissue distribution profiles were assessed in tumor-bearing mice. The efficacy of terbium-161 compared to lutetium-177 was investigated in therapy studies in mice using DOTATOC or DOTA-LM3, respectively.
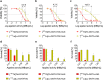
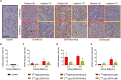
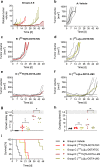
Results: In vitro, [161Tb]Tb-DOTA-LM3 was 102-fold more potent than [177Lu]Lu-DOTA-LM3; however, 161Tb-labeled DOTATOC and DOTATOC-NLS were only 4- to fivefold more effective inhibiting tumor cell viability than their 177Lu-labeled counterparts. This result was confirmed in vivo and demonstrated that [161Tb]Tb-DOTA-LM3 was significantly more effective in delaying tumor growth than [177Lu]Lu-DOTA-LM3, thereby, prolonging survival of the mice. A therapeutic advantage of terbium-161 over lutetium-177 was also manifest when applied with DOTATOC. Since the nuclear localizing sequence (NLS) compromised the in vivo tissue distribution of DOTATOC-NLS, it was not used for therapy.
Conclusion: The use of membrane-localizing DOTA-LM3 was beneficial and profited from the short-ranged electrons emitted by terbium-161. Based on these preclinical data, [161Tb]Tb-DOTA-LM3 may outperform the clinically employed [177Lu]Lu-DOTATOC for the treatment of patients with NENs.
Keywords: Auger electrons; NEN; PRRT; Radionuclide therapy; SSTR antagonists; Terbium-161.
© 2021. The Author(s).
Conflict of interest statement
Francesca Borgna, Roger Schibli, Nicholas P. van der Meulen, and Cristina Müller are co-inventors on a patent application filed by the Paul Scherrer Institute and the University Hospital Basel, Switzerland. All other authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

