Blocking Kallikrein 6 promotes developmental myelination
- PMID: 34626143
- PMCID: PMC8732303
- DOI: 10.1002/glia.24100
Blocking Kallikrein 6 promotes developmental myelination
Abstract
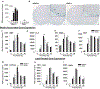
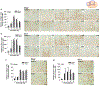
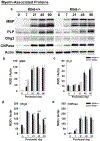
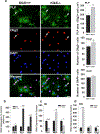
Kallikrein related peptidase 6 (Klk6) is a secreted serine protease highly expressed in oligodendrocytes and implicated in demyelinating conditions. To gain insights into the significance of Klk6 to oligodendrocyte biology, we investigated the impact of global Klk6 gene knockout on CNS developmental myelination using the spinal cord of male and female mice as a model. Results demonstrate that constitutive loss of Klk6 expression accelerates oligodendrocyte differentiation developmentally, including increases in the expression of myelin proteins such as MBP, PLP and CNPase, in the number of CC-1+ mature oligodendrocytes, and myelin thickness by the end of the first postnatal week. Co-ordinate elevations in the pro-myelinating signaling pathways ERK and AKT, expression of fatty acid 2-hydroxylase, and myelin regulatory transcription factor were also observed in the spinal cord of 7d Klk6 knockouts. LC/MS/MS quantification of spinal cord lipids showed sphingosine and sphingomyelins to be elevated in Klk6 knockouts at the peak of myelination. Oligodendrocyte progenitor cells (OPCs)-derived from Klk6 knockouts, or wild type OPCs-treated with a Klk6 inhibitor (DFKZ-251), also showed increased MBP and PLP. Moreover, inhibition of Klk6 in OPC cultures enhanced brain derived neurotrophic factor-driven differentiation. Altogether, these findings suggest that oligodendrocyte-derived Klk6 may operate as an autocrine or paracrine rheostat, or brake, on pro-myelinating signaling serving to regulate myelin homeostasis developmentally and in the adult. These findings document for the first time that inhibition of Klk6 globally, or specifically in oligodendrocyte progenitors, is a strategy to increase early stages of oligodendrocyte differentiation and myelin production in the CNS.
Keywords: brain derived neurotrophic factor; lipid; oligodendrocyte; spinal cord.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Figures





References
-
- Angelo PF, Lima AR, Alves FM, Blaber SI, Scarisbrick IA, Blaber M, Juliano L, Juliano MA. 2006. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan activation effects. J Biol Chem 281:3116–26. - PubMed
-
- Bando Y, Hagiwara Y, Suzuki Y, Yoshida K, Aburakawa Y, Kimura T, Murakami C, Ono M, Tanaka T, Jiang YP and others. 2018. Kallikrein 6 secreted by oligodendrocytes regulates the progression of experimental autoimmune encephalomyelitis. Glia 66:359–378. - PubMed
-
- Bando Y, Ito S, Nagai Y, Terayama R, Kishibe M, Jiang YP, Mitrovic B, Takahashi T, Yoshida S. 2006. Implications of protease M/neurosin in myelination during experimental demyelination and remyelination. Neurosci Lett 405:175–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

