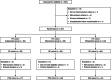
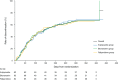
Discontinuation and remission rates and social functioning in patients with schizophrenia receiving second-generation antipsychotics: 52-week evaluation of JUMPs, a randomized, open-label study
- PMID: 34626144
- PMCID: PMC9299006
- DOI: 10.1111/pcn.13304
Discontinuation and remission rates and social functioning in patients with schizophrenia receiving second-generation antipsychotics: 52-week evaluation of JUMPs, a randomized, open-label study
Abstract
Aim: Globally, evidence from short-term studies is insufficient for the guidelines to uniformly recommend a particular antipsychotic(s) for the maintenance treatment of schizophrenia. Therefore, long-term comprehensive evaluation of antipsychotics is required from a social rehabilitation perspective, especially for drugs that have not yet been studied. The Japan Useful Medication Program for Schizophrenia (JUMPs) is a large-scale, long-term naturalistic study to present pivotal 52-week data on the continuity of second-generation antipsychotics (SGA: aripiprazole, blonanserin, and paliperidone).
Methods: JUMPs was an open-label, three-arm, randomized, parallel-group, 52-week study. Enrolled patients had schizophrenia, were ≥20 years old, and required antipsychotic treatment or switched from previous therapy. The primary endpoint was treatment discontinuation rate over 52 weeks. Secondary outcomes included remission rate, social functioning, and quality-of-life scores [Personal and Social Performance Scale (PSP) and EuroQol-5 dimensions], and safety.
Results: In total, 251 patients received aripiprazole (n = 82), blonanserin (n = 85), or paliperidone (n = 84). The discontinuation rate (P = 0.9771) and remission rates (P > 0.05) over 52 weeks did not differ significantly between the three treatment groups. The discontinuation rates were 68.3%, 68.2%, and 65.5% in the aripiprazole, blonanserin, and paliperidone groups, respectively. Significant improvements (all P < 0.05) from baseline in PSP scores were observed at start of monotherapy, week 26, and week 52 in the overall cohort and blonanserin group and at week 26 in the aripiprazole group. The adverse event profile favored blonanserin.
Conclusion: All three SGAs evaluated in this study showed similar treatment discontinuation rates in patients with chronic schizophrenia in Japan.
Keywords: discontinuation rate; long-term effectiveness; remission rate; second generation antipsychotics.
© 2021 The Authors. Psychiatry and Clinical Neurosciences published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Psychiatry and Neurology.
Figures
Similar articles
-
Randomized open-label study of second-generation antipsychotics for the treatment of schizophrenia: 104-week final results of the JUMPs study assessing treatment discontinuation, remission, and social functioning.BMC Psychiatry. 2024 Sep 5;24(1):600. doi: 10.1186/s12888-024-06031-4. BMC Psychiatry. 2024. PMID: 39237918 Free PMC article. Clinical Trial.
-
Japan useful medication program for schizophrenia (JUMPs)-long-term study on discontinuation rate, resolution and remission, and improvement in social functioning rate associated with atypical antipsychotic medications in patients with schizophrenia.BMC Psychiatry. 2013 Oct 3;13:243. doi: 10.1186/1471-244X-13-243. BMC Psychiatry. 2013. PMID: 24090047 Free PMC article. Clinical Trial.
-
Effect of aripiprazole on verbal memory and fluency in schizophrenic patients : results from the ESCAPE study.CNS Drugs. 2012 Nov;26(11):975-82. doi: 10.1007/s40263-012-0003-4. CNS Drugs. 2012. PMID: 23018547 Clinical Trial.
-
Efficacy and tolerability of blonanserin in schizophrenia: A systematic review and meta-analysis of randomized controlled trials.Schizophr Res. 2024 Dec;274:360-373. doi: 10.1016/j.schres.2024.10.016. Epub 2024 Oct 28. Schizophr Res. 2024. PMID: 39490217
-
Efficacy, Tolerability, and Safety of Blonanserin in Schizophrenia: An Updated and Extended Systematic Review and Meta-Analysis of Randomized Controlled Trials.Pharmacopsychiatry. 2019 Feb;52(2):52-62. doi: 10.1055/a-0574-0088. Epub 2018 Mar 7. Pharmacopsychiatry. 2019. PMID: 29514360
Cited by
-
Meta-Analysis of the Effect of Blonanserin in Treating Patients with Schizophrenia.Noro Psikiyatr Ars. 2025 Jun 11;62(2):195-204. doi: 10.29399/npa.28774. eCollection 2025. Noro Psikiyatr Ars. 2025. PMID: 40583941 Free PMC article. Review.
-
Non-adherence and predictors in patients with schizophrenia on second generation antipsychotics at Amanuel Mental Specialized Hospital, Ethiopia.PLoS One. 2025 Mar 26;20(3):e0314403. doi: 10.1371/journal.pone.0314403. eCollection 2025. PLoS One. 2025. PMID: 40138309 Free PMC article.
-
Effectiveness and safety of blonanserin in young and middle-aged female patients with schizophrenia: data from a post-marketing surveillance.BMC Psychiatry. 2023 Feb 21;23(1):115. doi: 10.1186/s12888-023-04598-y. BMC Psychiatry. 2023. PMID: 36810039 Free PMC article.
-
Randomized open-label study of second-generation antipsychotics for the treatment of schizophrenia: 104-week final results of the JUMPs study assessing treatment discontinuation, remission, and social functioning.BMC Psychiatry. 2024 Sep 5;24(1):600. doi: 10.1186/s12888-024-06031-4. BMC Psychiatry. 2024. PMID: 39237918 Free PMC article. Clinical Trial.
-
A multicenter, single-arm, open-label interventional study of adherence to brexpiprazole during switching from previous antipsychotic drugs in patients with schizophrenia or schizoaffective disorder.Neuropsychopharmacol Rep. 2024 Mar;44(1):187-196. doi: 10.1002/npr2.12416. Epub 2024 Jan 22. Neuropsychopharmacol Rep. 2024. PMID: 38253334 Free PMC article. Clinical Trial.
References
-
- The Ministry of Health, Labour and Welfare. Statistics by Ministry of Health, Labour and Welfare, as of 2008. [Cited 3 November 2020] Available from URL: http://www.mhlw.go.jp/kokoro/speciality/detail_into.html.
-
- National Institute for Health and Care Excellence. NICE guideline 2014. Psychosis and schizophrenia in adults: prevention and management. [Cited 3 November 2020] Available from URL: https://www.nice.org.uk/guidance/cg178/chapter/1‐Recommendations#promoti.... - PubMed
-
- American Psychiatric Association . APA practical guideline 2019. [Cited 3 November 2020] Available from URL: https://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2020.177901. https://www.psychiatry.org/psychiatrists/practice/clinical‐practice‐guid... - DOI
-
- The Japanese Society of Neuropsychopharmacology. “Guideline for Pharmacological Therapy of Schizophrenia” [Cited 3 November 2020] Available from URL: http://www.asas.or.jp/jsnp/csrinfo/03.html. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous