Convolutional Neural Network Based Frameworks for Fast Automatic Segmentation of Thalamic Nuclei from Native and Synthesized Contrast Structural MRI
- PMID: 34626333
- PMCID: PMC8993941
- DOI: 10.1007/s12021-021-09544-5
Convolutional Neural Network Based Frameworks for Fast Automatic Segmentation of Thalamic Nuclei from Native and Synthesized Contrast Structural MRI
Abstract
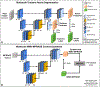
Thalamic nuclei have been implicated in several neurological diseases. Thalamic nuclei parcellation from structural MRI is challenging due to poor intra-thalamic nuclear contrast while methods based on diffusion and functional MRI are affected by limited spatial resolution and image distortion. Existing multi-atlas based techniques are often computationally intensive and time-consuming. In this work, we propose a 3D convolutional neural network (CNN) based framework for thalamic nuclei parcellation using T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) images. Transformation of images to an efficient representation has been proposed to improve the performance of subsequent classification tasks especially when working with limited labeled data. We investigate this by transforming the MPRAGE images to White-Matter-nulled MPRAGE (WMn-MPRAGE) contrast, previously shown to exhibit good intra-thalamic nuclear contrast, prior to the segmentation step. We trained two 3D segmentation frameworks using MPRAGE images (n = 35 subjects): (a) a native contrast segmentation (NCS) on MPRAGE images and (b) a synthesized contrast segmentation (SCS) where synthesized WMn-MPRAGE representation generated by a contrast synthesis CNN were used. Thalamic nuclei labels were generated using THOMAS, a multi-atlas segmentation technique proposed for WMn-MPRAGE images. The segmentation accuracy and clinical utility were evaluated on a healthy cohort (n = 12) and a cohort (n = 45) comprising of healthy subjects and patients with alcohol use disorder (AUD), respectively. Both the segmentation CNNs yielded comparable performances on most thalamic nuclei with Dice scores greater than 0.84 for larger nuclei and at least 0.7 for smaller nuclei. However, for some nuclei, the SCS CNN yielded significant improvements in Dice scores (medial geniculate nucleus, P = 0.003, centromedian nucleus, P = 0.01) and percent volume difference (ventral anterior, P = 0.001, ventral posterior lateral, P = 0.01) over NCS. In the AUD cohort, the SCS CNN demonstrated a significant atrophy in ventral lateral posterior nucleus in AUD patients compared to healthy age-matched controls (P = 0.01), agreeing with previous studies on thalamic atrophy in alcoholism, whereas the NCS CNN showed spurious atrophy of the ventral posterior lateral nucleus. CNN-based segmentation of thalamic nuclei provides a fast and automated technique for thalamic nuclei prediction in MPRAGE images. The transformation of images to an efficient representation, such as WMn-MPRAGE, can provide further improvements in segmentation performance.
Keywords: 3D convolutional neural networks; Alcohol use disorder; Contrast synthesis; Representational learning; Thalamic-nuclei segmentation; White-matter-nulled MPRAGE.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflict of Interest
The authors have no conflicts of interest, relevant to this manuscript, to disclose. Additional disclosures: MBK (spouse of LU) is an employee of Siemens Medical Solutions, USA.
Figures



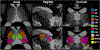

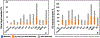

Similar articles
-
Automated thalamic nuclei segmentation using multi-planar cascaded convolutional neural networks.Magn Reson Imaging. 2020 Nov;73:45-54. doi: 10.1016/j.mri.2020.08.005. Epub 2020 Aug 21. Magn Reson Imaging. 2020. PMID: 32828985 Free PMC article.
-
Fast automatic segmentation of thalamic nuclei from MP2RAGE acquisition at 7 Tesla.Magn Reson Med. 2021 May;85(5):2781-2790. doi: 10.1002/mrm.28608. Epub 2020 Dec 3. Magn Reson Med. 2021. PMID: 33270943
-
Robust thalamic nuclei segmentation from T1-weighted MRI using polynomial intensity transformation.Brain Struct Funct. 2024 Jun;229(5):1087-1101. doi: 10.1007/s00429-024-02777-5. Epub 2024 Mar 28. Brain Struct Funct. 2024. PMID: 38546872 Free PMC article.
-
Performance of Convolutional Neural Network Models in Meningioma Segmentation in Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis.Neuroinformatics. 2025 Jan;23(1):14. doi: 10.1007/s12021-024-09704-3. Epub 2024 Dec 28. Neuroinformatics. 2025. PMID: 39777602 Free PMC article.
-
Unlocking the Power of 3D Convolutional Neural Networks for COVID-19 Detection: A Comprehensive Review.J Imaging Inform Med. 2025 Jan 23. doi: 10.1007/s10278-025-01393-x. Online ahead of print. J Imaging Inform Med. 2025. PMID: 39849202 Review.
Cited by
-
Accurate Bayesian segmentation of thalamic nuclei using diffusion MRI and an improved histological atlas.Neuroimage. 2023 Jul 1;274:120129. doi: 10.1016/j.neuroimage.2023.120129. Epub 2023 Apr 22. Neuroimage. 2023. PMID: 37088323 Free PMC article.
-
Multi-atlas thalamic nuclei segmentation on standard T1-weighed MRI with application to normal aging.Hum Brain Mapp. 2023 Feb 1;44(2):612-628. doi: 10.1002/hbm.26088. Epub 2022 Oct 1. Hum Brain Mapp. 2023. PMID: 36181510 Free PMC article.
-
Thalamic atrophy in multiple sclerosis is associated with tract disconnection and altered microglia.Acta Neuropathol. 2025 May 28;149(1):52. doi: 10.1007/s00401-025-02893-4. Acta Neuropathol. 2025. PMID: 40434526 Free PMC article.
-
A roadmap towards standardized neuroimaging approaches for human thalamic nuclei.Nat Rev Neurosci. 2024 Dec;25(12):792-808. doi: 10.1038/s41583-024-00867-1. Epub 2024 Oct 17. Nat Rev Neurosci. 2024. PMID: 39420114 Review.
-
Thalamic nuclei segmentation from T1-weighted MRI: Unifying and benchmarking state-of-the-art methods.Imaging Neurosci (Camb). 2024 May 8;2:1-16. doi: 10.1162/imag_a_00166. eCollection 2024 May 1. Imaging Neurosci (Camb). 2024. PMID: 40041300 Free PMC article.
References
-
- Battistella Giovanni, Najdenovska Elena, Maeder Philippe, Ghazaleh Naghmeh, Daducci Alessandro, Thiran Jean Philippe, Jacquemont Sébastien, et al. 2017. “Robust Thalamic Nuclei Segmentation Method Based on Local Diffusion Magnetic Resonance Properties.” Brain Structure and Function 222 (5): 2203–16. 10.1007/s00429-016-1336-4. - DOI - PMC - PubMed
-
- Bernstein Adam S, Rapcsak Steven Z, Hornberger Michael, Saranathan Manojkumar, and the Alzheimer’s Disease Neuroimaging Initiative. 2021. “Structural Changes in Thalamic Nuclei Across Prodromal and Clinical Alzheimer’s Disease.” Journal of Alzheimer’s Disease 82: 361–71. 10.3233/JAD-201583. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

