The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin
- PMID: 34626339
- PMCID: PMC8501344
- DOI: 10.1007/s40265-021-01611-0
The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin
Abstract
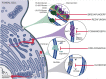
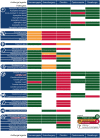
The epidemiology of invasive fungal infections is changing, with new populations at risk and the emergence of resistance caused by the selective pressure from increased usage of antifungal agents in prophylaxis, empiric therapy, and agriculture. Limited antifungal therapeutic options are further challenged by drug-drug interactions, toxicity, and constraints in administration routes. Despite the need for more antifungal drug options, no new classes of antifungal drugs have become available over the last 2 decades, and only one single new agent from a known antifungal class has been approved in the last decade. Nevertheless, there is hope on the horizon, with a number of new antifungal classes in late-stage clinical development. In this review, we describe the mechanisms of drug resistance employed by fungi and extensively discuss the most promising drugs in development, including fosmanogepix (a novel Gwt1 enzyme inhibitor), ibrexafungerp (a first-in-class triterpenoid), olorofim (a novel dihyroorotate dehydrogenase enzyme inhibitor), opelconazole (a novel triazole optimized for inhalation), and rezafungin (an echinocandin designed to be dosed once weekly). We focus on the mechanism of action and pharmacokinetics, as well as the spectrum of activity and stages of clinical development. We also highlight the potential future role of these drugs and unmet needs.
© 2021. The Author(s).
Conflict of interest statement
MH has received research funding from Gilead, Astellas, Pfizer, Merck, Scynexis, F2G, Pulmocide and Amplyx. RS has nothing to disclose. ME has nothing to disclose. AA has nothing to disclose. OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, and Scynexis; consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, and Seres; honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, and Pfizer; payment for expert testimony from Cidara; participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI, and Shionogi; a pending patent currently reviewed at the German Patent and Trade Mark Office; other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, and Wiley. RK has nothing to disclose. CLF reports grants, consulting fees, support for travel to meetings and payment for lectures including service on speakers bureaus from Gilead Sciences, Astellas Pharma, Merck Sharp and Dahme, Basilea, and Angelini. JP has nothing to disclose. AS has received grant funding from Astellas and Mayne, and consulting fees from Scynexis and Mayne. GRT has received honoraria from Amplyx, Cidara, Mayne Pharma, Pfizer, and Scynexis. NW has received research funding from Astellas, bioMerieux, Cepheid, Covance, F2G, and Sfunga, and has served on an advisory board for Mayne Pharma. JDJ has received research funding from Astellas, Pfizer, and F2G.
Figures

References
-
- Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. - DOI - PMC - PubMed
-
- Hoenigl M, Salmanton-García J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis. 2021;21(8):e246–e257. doi: 10.1016/S1473-3099(20)30784-2. - DOI - PubMed
-
- Salmanton-GarcÍa J, Koehler P, Kindo A, Falces-Romero I, GarcÍa-RodrÍguez J, RÁČil Z, et al. Needles in a haystack: extremely rare invasive fungal infections reported in FungiScope®—Global Registry for Emerging Fungal Infections. J Infect. 2020;81(5):802–815. doi: 10.1016/j.jinf.2020.08.015. - DOI - PubMed
-
- Jenks JD, Seidel D, Cornely OA, Chen S, van Hal S, Kauffman C, et al. Voriconazole plus terbinafine combination antifungal therapy for invasive Lomentospora prolificans infections: analysis of 41 patients from the FungiScope® registry 2008-2019. Clin Microbiol Infect. 2020;S1198-743X(20)30037-9. - PubMed
-
- Arastehfar A, de Almeida Júnior JN, Perlin DS, Ilkit M, Boekhout T, Colombo AL. Multidrug-resistant Trichosporon species: underestimated fungal pathogens posing imminent threats in clinical settings. Crit Rev Microbiol. 2021:1–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

