Gender, age, disease severity, body mass index and diabetes may not affect response to subcutaneous tanezumab in patients with osteoarthritis after 16 weeks of treatment. A subgroup analysis of placebo-controlled trials
- PMID: 34626502
- PMCID: PMC9285028
- DOI: 10.1111/ijcp.14975
Gender, age, disease severity, body mass index and diabetes may not affect response to subcutaneous tanezumab in patients with osteoarthritis after 16 weeks of treatment. A subgroup analysis of placebo-controlled trials
Abstract
Aim: To assess the impact of pre-specified patient characteristics on efficacy and safety of subcutaneous tanezumab in patients with osteoarthritis (OA).
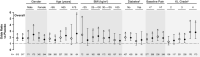
Methods: Data were pooled from two (efficacy; N = 1545) or three (safety; N = 1754) phase 3 placebo-controlled trials. Change from baseline to week 16 in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain, WOMAC Physical Function and patient global assessment of OA (PGA-OA) scores was compared between tanezumab (2.5 and 5 mg) and placebo groups via analysis of covariance. Treatment-emergent adverse events (TEAEs) were summarised descriptively. Analyses were done in patient subgroups (men or women; age <65, ≥65, or ≥75 years; body mass index [BMI] <25, 25 to <30, 30 to <35 or ≥35 kg/m2 ; diabetes or no diabetes; baseline WOMAC Pain score <7 or ≥7; and Kellgren-Lawrence [KL] grades 2, 3 or 4 in the index joint) and the overall population.
Results: In all subgroups, improvements in WOMAC Pain were numerically greater and often statistically significant (P < .05) for both tanezumab groups compared with placebo. Results were similar for WOMAC Physical Function and PGA-OA. TEAE profiles were generally consistent across subgroups and similar to the overall population (ie slightly higher rates of TEAEs, serious TEAEs and severe TEAEs with tanezumab relative to placebo) with a few exceptions. Exceptions included women reporting slightly more TEAEs with tanezumab than men, and patients with diabetes reporting slightly more severe TEAEs with tanezumab than patients without diabetes. Additionally, TEAEs were more frequent with tanezumab than placebo in the age ≥65 and ≥75 years, but not the age <65 years, subgroups.
Conclusions: Efficacy and safety/tolerability of tanezumab may not be meaningfully impacted by gender, age, BMI, diabetes status, baseline pain severity or KL grade in the index joint. Conclusions are limited by low patient number in some subgroups. Clinicaltrials.gov: NCT02697773, NCT02709486, NCT01089725.
© 2021 Pfizer Inc. International Journal of Clinical Practice published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients With Osteoarthritis of the Hip or Knee: A Randomized Clinical Trial.JAMA. 2019 Jul 2;322(1):37-48. doi: 10.1001/jama.2019.8044. JAMA. 2019. PMID: 31265100 Free PMC article. Clinical Trial.
-
Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period.Ann Rheum Dis. 2020 Jun;79(6):800-810. doi: 10.1136/annrheumdis-2019-216296. Epub 2020 Mar 31. Ann Rheum Dis. 2020. PMID: 32234715 Free PMC article. Clinical Trial.
-
A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee.Pain. 2013 Sep;154(9):1603-1612. doi: 10.1016/j.pain.2013.04.035. Epub 2013 Apr 22. Pain. 2013. PMID: 23707270 Clinical Trial.
-
Efficacy and safety of tanezumab administered as a fixed dosing regimen in patients with knee or hip osteoarthritis: a meta-analysis of randomized controlled phase III trials.Clin Rheumatol. 2021 Jun;40(6):2155-2165. doi: 10.1007/s10067-020-05488-4. Epub 2020 Nov 6. Clin Rheumatol. 2021. PMID: 33159281 Review.
-
Tanezumab for Patients with Osteoarthritis of the Knee: A Meta-Analysis.PLoS One. 2016 Jun 13;11(6):e0157105. doi: 10.1371/journal.pone.0157105. eCollection 2016. PLoS One. 2016. PMID: 27294371 Free PMC article. Review.
Cited by
-
Sexual dimorphism in peri-articular tissue anatomy - More keys to understanding sex-differences in osteoarthritis?Osteoarthr Cartil Open. 2024 May 11;6(3):100485. doi: 10.1016/j.ocarto.2024.100485. eCollection 2024 Sep. Osteoarthr Cartil Open. 2024. PMID: 38946793 Free PMC article. Review.
References
-
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323‐1330. - PubMed
-
- Geenen R, Overman CL, Christensen R, et al. EULAR recommendations for the health professional's approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77:797‐807. - PubMed
-
- Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non‐surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578‐1589. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
