The Lancet Commission on diagnostics: transforming access to diagnostics
- PMID: 34626542
- PMCID: PMC8494468
- DOI: 10.1016/S0140-6736(21)00673-5
The Lancet Commission on diagnostics: transforming access to diagnostics
Erratum in
-
Department of Error.Lancet. 2021 Nov 27;398(10315):1964. doi: 10.1016/S0140-6736(21)02275-3. Epub 2021 Nov 8. Lancet. 2021. PMID: 34762858 Free PMC article. No abstract available.
Conflict of interest statement
Declaration of interests TD declares minor shareholdings and an unpaid directorship at CapeRay Medical, which was an institution-based activity. WC declares he is the chief executive officer of a telemedicine company providing general medical consultation. All other authors declare no competing interests.
Figures
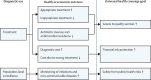
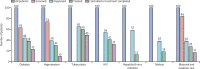

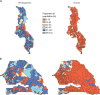
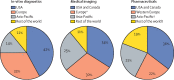



Comment in
-
Can COVID-19 help accelerate and transform the diagnostics agenda?Lancet. 2021 Nov 27;398(10315):1945-1947. doi: 10.1016/S0140-6736(21)02093-6. Epub 2021 Oct 6. Lancet. 2021. PMID: 34626541 Free PMC article. No abstract available.
-
Transforming access to diagnostics: how to turn good intentions into action?Lancet. 2021 Nov 27;398(10315):1947-1949. doi: 10.1016/S0140-6736(21)02182-6. Epub 2021 Oct 6. Lancet. 2021. PMID: 34626543 Free PMC article. No abstract available.
-
Expanding diagnostics for LMICs.Lancet. 2022 Apr 23;399(10335):1604-1605. doi: 10.1016/S0140-6736(22)00336-1. Lancet. 2022. PMID: 35461551 No abstract available.
-
Transforming the UK's diagnostics agenda after COVID-19.Lancet. 2022 Apr 23;399(10335):1606. doi: 10.1016/S0140-6736(22)00169-6. Lancet. 2022. PMID: 35461555 Free PMC article. No abstract available.
References
-
- US Food and Drug Administration Coronavirus (COVID-19) update: FDA alerts consumers about unauthorized fraudulent COVID-19 test kits. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
-
- Shuren J, Stenzel T. The FDA's experience with Covid-19 antibody tests. N Engl J Med. 2021;384:592–594. - PubMed
-
- Chmura C, Jackson J. Feds work to snag fake COVID-19 test kits, bogus virus products. 2020. https://www.nbcbayarea.com/investigations/consumer/feds-work-to-snag-fak...
-
- The Global Fund Access to COVID-19 Tools Accelerator. 2021. https://www.theglobalfund.org/en/covid-19/act-a/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

