Chronic intermittent ethanol during adolescence and adulthood alters dendritic spines in the primary motor and visual cortex in rats
- PMID: 34626787
- PMCID: PMC9671089
- DOI: 10.1016/j.alcohol.2021.09.032
Chronic intermittent ethanol during adolescence and adulthood alters dendritic spines in the primary motor and visual cortex in rats
Abstract
Prolonged adolescent binge drinking can disrupt sleep quality and increase the likelihood of alcohol-induced sleep disruptions in young adulthood in rodents and in humans. Striking changes in spine density and morphology have been seen in many cortical and subcortical regions after adolescent alcohol exposure in rats. However, there is little known about the impact of alcohol exposure on dendritic spines in the same motor and sensory cortices that EEG sleep is typically recorded from in rats. The aim of this study is to investigate whether an established model of chronic intermittent ethanol vapor in rats that has been demonstrated to disrupt sleep during adolescence or adulthood, also significantly alters cortical dendritic spine density and morphology. To this end, adolescent and adult Wistar rats were exposed to 5 weeks of ethanol vapor or control air exposure. After a 13-day withdrawal, primary motor cortex (M1) and primary/secondary visual cortex (V1/V2) layer V dendrites were analyzed for differences in spine density and morphology. Spines were classified into four categories (stubby, long, filopodia, and mushroom) based on the spine length and the width of the spine head and neck. The main results indicate an age-specific effect of adolescent intermittent ethanol exposure decreasing spine density in the M1 cortex compared to age-matched controls. Reductions in the density of M1 long-shaped spine subclassifications were seen in adolescent ethanol-exposed rats, but not adult-exposed rats, compared to their air-controls. Irrespective of age, there was an overall reduction produced by ethanol exposure on the density of filopodia and the length of long-shaped spines in V1/V2 cortex as compared to their air-exposed controls. Together, these data add to growing evidence that some cortical circuits are vulnerable to the effects of alcohol during adolescence and begin to elucidate potential mechanisms that may influence brain plasticity following early alcohol use.
Keywords: Adolescence; Alcohol; Dendrites; Motor cortex; Sleep; Spines; Visual cortex.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures

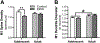
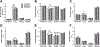
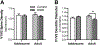
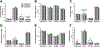
Similar articles
-
Chronic Ethanol During Adolescence Impacts Corticolimbic Dendritic Spines and Behavior.Alcohol Clin Exp Res. 2017 Jul;41(7):1298-1308. doi: 10.1111/acer.13422. Epub 2017 Jun 14. Alcohol Clin Exp Res. 2017. PMID: 28614590 Free PMC article.
-
Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice.Alcohol. 2015 Feb;49(1):21-7. doi: 10.1016/j.alcohol.2014.07.017. Epub 2014 Nov 3. Alcohol. 2015. PMID: 25468278 Free PMC article.
-
Contrasting effects of adolescent and early-adult ethanol exposure on prelimbic cortical pyramidal neurons.Drug Alcohol Depend. 2020 Nov 1;216:108309. doi: 10.1016/j.drugalcdep.2020.108309. Epub 2020 Sep 21. Drug Alcohol Depend. 2020. PMID: 32998090 Free PMC article.
-
Neuroepigenetic consequences of adolescent ethanol exposure.Int Rev Neurobiol. 2021;160:45-84. doi: 10.1016/bs.irn.2021.06.008. Epub 2021 Jul 27. Int Rev Neurobiol. 2021. PMID: 34696879 Review.
-
Areas of Convergence and Divergence in Adolescent Social Isolation and Binge Drinking: A Review.Front Behav Neurosci. 2022 Mar 30;16:859239. doi: 10.3389/fnbeh.2022.859239. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35431830 Free PMC article. Review.
Cited by
-
Effects of daridorexant on rest/wake activity patterns and drinking in adult rats exposed to chronic ethanol vapor in adolescence.Alcohol. 2025 May;124:35-46. doi: 10.1016/j.alcohol.2025.01.006. Epub 2025 Jan 25. Alcohol. 2025. PMID: 39870333
-
Ethanol-Induced Depression: Exploring the Underlying Molecular Mechanisms.Cell Mol Neurobiol. 2025 May 22;45(1):49. doi: 10.1007/s10571-025-01569-7. Cell Mol Neurobiol. 2025. PMID: 40405002 Free PMC article. Review.
-
Single-dose ethanol intoxication causes acute and lasting neuronal changes in the brain.Proc Natl Acad Sci U S A. 2022 Jun 21;119(25):e2122477119. doi: 10.1073/pnas.2122477119. Epub 2022 Jun 14. Proc Natl Acad Sci U S A. 2022. PMID: 35700362 Free PMC article.
-
Effects of Chronic Social Isolation Stress and Alcohol on the Reinforcing Properties of Ketamine in Male and Female Rats.eNeuro. 2025 Mar 3;12(3):ENEURO.0452-24.2025. doi: 10.1523/ENEURO.0452-24.2025. Print 2025 Mar. eNeuro. 2025. PMID: 39993843 Free PMC article.
-
Effect of chronic sleep restriction on ethanol preference and cortical structural plasticity.Neurobiol Sleep Circadian Rhythms. 2025 May 14;18(Suppl):100126. doi: 10.1016/j.nbscr.2025.100126. eCollection 2025 May. Neurobiol Sleep Circadian Rhythms. 2025. PMID: 40703573 Free PMC article.
References
-
- Amodeo LR, Wills DN, Sanchez-Alavez M, & Ehlers CL (2020). Effects of orexin-2 receptor antagonist on sleep and event-related oscillations in female rats exposed to chronic intermittent ethanol during adolescence. Alcoholism: Clinical and Experimental Research. 10.1111/acer.14361 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

