Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo
- PMID: 34626854
- PMCID: PMC8590079
- DOI: 10.1016/j.molmet.2021.101352
Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo
Abstract
Objective: Long-acting glucagon-like peptide-1 receptor agonists (GLP-1RAs), like liraglutide and semaglutide, are viable treatments for diabetes and obesity. Liraglutide directly activates hypothalamic proopiomelanocortin (POMC) neurons while indirectly inhibiting Neuropeptide Y/Agouti-related peptide (NPY/AgRP) neurons ex vivo. While temporal control of GLP-1R agonist concentration as well as accessibility to tissues/cells can be achieved with relative ease ex vivo, in vivo this is dependent upon the pharmacokinetics of these agonists and relative penetration into structures of interest. Thus, whether liraglutide or semaglutide modifies the activity of POMC and NPY/AgRP neurons in vivo as well as mechanisms required for any changes in cellular activity remains undefined.
Methods: In order to resolve this issue, we utilized neuron-specific transgenic mouse models to examine changes in the activity of POMC and NPY/AgRP neurons after injection of either liraglutide or semaglutide (intraperitoneal - I.P. and subcutaneous - S·C.). POMC and NPY/AgRP neurons were targeted for patch-clamp electrophysiology as well as in vivo fiber photometry.
Results: We found that liraglutide and semaglutide directly activate and increase excitatory tone to POMC neurons in a time-dependent manner. This increased activity of POMC neurons required GLP-1Rs in POMC neurons as well as a downstream mixed cation channel comprised of TRPC5 subunits. We also observed an indirect upregulation of excitatory input to POMC neurons originating from glutamatergic cells that also required TRPC5 subunits. Conversely, GLP-1Ra's decreased excitatory input to and indirectly inhibited NPY/AgRP neurons through activation of K-ATP and TRPC5 channels in GABAergic neurons. Notably, the temporal activation of POMC and inhibition of NPY/AgRP neuronal activity after liraglutide or semaglutide was injected [either intraperitoneal (I.P.) or subcutaneous (S·C.)] was dependent upon the nutritional state of the animals (fed vs food-deprived).
Conclusions: Our results support a mechanism of liraglutide and semaglutide in vivo to activate POMC while inhibiting NPY/AgRP neurons, which depends upon metabolic state and mirrors the pharmacokinetic profile of these compounds in vivo.
Keywords: GLP-1R Agonists on NPY/AgRP neuron; Liraglutide and semaglutide; Long-acting glucagon-like peptide-1 receptor agonists (GLP-1RAs); Metabolic state-dependent effects; POMC Neuronal activity; TRPC5 subunit.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures





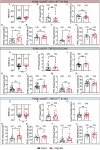

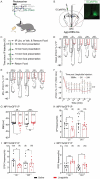

References
-
- Kreymann B., Williams G., Ghatei M.A., Bloom S.R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. - PubMed
-
- Larsen P.J., Tang-Christensen M., Holst J.J., Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. - PubMed
-
- Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27:740–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

