GPR65 (TDAG8) inhibits intestinal inflammation and colitis-associated colorectal cancer development in experimental mouse models
- PMID: 34628032
- PMCID: PMC8629932
- DOI: 10.1016/j.bbadis.2021.166288
GPR65 (TDAG8) inhibits intestinal inflammation and colitis-associated colorectal cancer development in experimental mouse models
Abstract
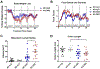
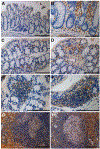
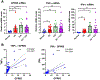
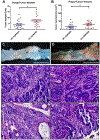
GPR65 (TDAG8) is a proton-sensing G protein-coupled receptor predominantly expressed in immune cells. Genome-wide association studies (GWAS) have identified GPR65 gene polymorphisms as an emerging risk factor for the development of inflammatory bowel disease (IBD). Patients with IBD have an elevated risk of developing colorectal cancer when compared to the general population. To study the role of GPR65 in intestinal inflammation and colitis-associated colorectal cancer (CAC), colitis and CAC were induced in GPR65 knockout (KO) and wild-type (WT) mice using dextran sulfate sodium (DSS) and azoxymethane (AOM)/DSS, respectively. Disease severity parameters such as fecal score, colon shortening, histopathology, and mesenteric lymph node enlargement were aggravated in GPR65 KO mice compared to WT mice treated with DSS. Elevated leukocyte infiltration and fibrosis were observed in the inflamed colon of GPR65 KO when compared to WT mice which may represent a cellular mechanism for the observed exacerbation of intestinal inflammation. In line with high expression of GPR65 in infiltrated leukocytes, GPR65 gene expression was increased in inflamed intestinal tissue samples of IBD patients compared to normal intestinal tissues. Moreover, colitis-associated colorectal cancer development was higher in GPR65 KO mice than WT mice when treated with AOM/DSS. Altogether, our data demonstrate that GPR65 suppresses intestinal inflammation and colitis-associated tumor development in murine colitis and CAC models, suggesting potentiation of GPR65 with agonists may have an anti-inflammatory therapeutic effect in IBD and reduce the risk of developing colitis-associated colorectal cancer.
Keywords: Colorectal cancer; GPR65 (TDAG8); Inflammatory bowel disease (IBD).
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest
None.
Figures





References
-
- Al-Mossawi MH, Chen L, Fang H, Ridley A, de Wit J, Yager N, Hammitzsch A, Pulyakhina I, Fairfax BP, Simone D, Yi Y, Bandyopadhyay S, Doig K, Gundle R, Kendrick B, Powrie F, Knight JC, Bowness P, Unique transcriptome signatures and GM-CSF expression in lymphocytes from patients with spondyloarthritis, Nat Commun 8 (2017) 1510. - PMC - PubMed
-
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH, Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease, Nature 491 (2012) 119–124. - PMC - PubMed
-
- Lahue KG, Lara MK, Linton AA, Lavoie B, Fang Q, McGill MM, Crothers JW, Teuscher C, Mawe GM, Tyler AL, Mahoney JM, Krementsov DN, Identification of novel loci controlling inflammatory bowel disease susceptibility utilizing the genetic diversity of wild-derived mice, Genes and immunity 21 (2020) 311–325. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

