Neuropeptide Y attenuates cardiac remodeling and deterioration of function following myocardial infarction
- PMID: 34628054
- PMCID: PMC8821956
- DOI: 10.1016/j.ymthe.2021.10.005
Neuropeptide Y attenuates cardiac remodeling and deterioration of function following myocardial infarction
Erratum in
-
Neuropeptide Y attenuates cardiac remodeling and deterioration of function following myocardial infarction.Mol Ther. 2024 Feb 7;32(2):559-561. doi: 10.1016/j.ymthe.2023.12.013. Epub 2023 Dec 19. Mol Ther. 2024. PMID: 38118444 Free PMC article. No abstract available.
Abstract
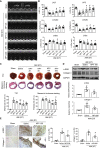
Plasma levels of neuropeptide Y (NPY) are elevated in patients with acute myocardial infarction (AMI), but its role in AMI remains unclear, which was examined here in NPY wild-type/knockout (WT/KO) mice treated with/without exogenous NPY and its Y1 receptor antagonist (Y1Ra) BIBP 3226. We found that AMI mice lacking NPY developed more severe AMI than WT mice with worse cardiac dysfunction, progressive cardiac inflammation and fibrosis, and excessive apoptosis but impairing angiogenesis. All of these changes were reversed when the NPY KO mice were treated with exogenous NPY in a dose-dependent manner. Interestingly, treatment with NPY also dose dependently attenuated AMI in WT mice, which was blocked by BIBP 3226. Phenotypically, cardiac NPY was de novo expressed by infiltrating macrophages during the repairing or fibrosing process in heart-failure patients and AMI mice. Mechanistically, NPY was induced by transforming growth factor (TGF)-β1 in bone marrow-derived macrophages and signaled through its Y1R to exert its pathophysiological activities by inhibiting p38/nuclear factor κB (NF-κB)-mediated M1 macrophage activation while promoting the reparative M2 phenotype in vivo and in vitro. In conclusion, NPY can attenuate AMI in mice. Inhibition of cardiac inflammation and fibrosis while enhancing angiogenesis but reducing apoptosis may be the underlying mechanisms through which NPY attenuates cardiac remodeling and deterioration of function following AMI.
Keywords: AMI; NPY; Y1 receptor; cardiac remodeling; macrophage.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures








References
-
- Suter L.G., Li S.X., Grady J.N., Lin Z., Wang Y., Bhat K.R., Turkmani D., Spivack S.B., Lindenauer P.K., Merrill A.R., et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J. Gen. Intern. Med. 2014;29:1333–1340. - PMC - PubMed
-
- Lewis E.F., Moye L.A., Rouleau J.L., Sacks F.M., Arnold J.M.O., Warnica J.W., Flaker G.C., Braunwald E., Pfeffer M.A., CARE Study Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J. Am. Coll. Cardiol. 2003;42:1446–1453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

