AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice
- PMID: 34628484
- PMCID: PMC8502176
- DOI: 10.1038/s41419-021-04184-8
AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice
Abstract
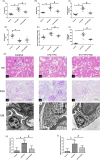
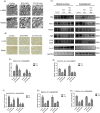
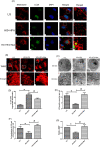
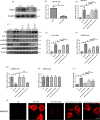
Renal tubulointerstitial fibrosis was a crucial pathological feature of diabetic nephropathy (DN), and renal tubular injury might associate with abnormal mitophagy. In this study, we investigated the effects and molecular mechanisms of AMPK agonist metformin on mitophagy and cellular injury in renal tubular cell under diabetic condition. The high fat diet (HFD) and streptozotocin (STZ)-induced type 2 diabetic mice model and HK-2 cells were used in this study. Metformin was administered in the drinking water (200 mg/kg/d) for 24 weeks. Renal tubulointerstitial lesions, oxidative stress and some indicators of mitophagy (e.g., LC3II, Pink1, and Parkin) were examined both in renal tissue and HK-2 cells. Additionally, compound C (an AMPK inhibitor) and Pink1 siRNA were applied to explore the molecular regulation mechanism of metformin on mitophagy. We found that the expression of p-AMPK, Pink1, Parkin, LC3II, and Atg5 in renal tissue of diabetic mice was decreased obviously. Metformin reduced the levels of serum creatinine, urine protein, and attenuated renal oxidative injury and fibrosis in HFD/STZ induced diabetic mice. In addition, Metformin reversed mitophagy dysfunction and the over-expression of NLRP3. In vitro pretreatment of HK-2 cells with AMPK inhibitor compound C or Pink1 siRNA negated the beneficial effects of metformin. Furthermore, we noted that metformin activated p-AMPK and promoted the translocation of Pink1 from the cytoplasm to mitochondria, then promoted the occurrence of mitophagy in HK-2 cells under HG/HFA ambience. Our results suggested for the first time that AMPK agonist metformin ameliorated renal oxidative stress and tubulointerstitial fibrosis in HFD/STZ-induced diabetic mice via activating mitophagy through a p-AMPK-Pink1-Parkin pathway.
© 2021. The Author(s).
Conflict of interest statement
The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
The animal experiments was approved by the Ethics Review Committee of The Third Xiangya Hospital, Central South University. The authors declare that they have no conflict of interest
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

