Pharmacotherapy for hypertension-induced left ventricular hypertrophy
- PMID: 34628642
- PMCID: PMC8502530
- DOI: 10.1002/14651858.CD012039.pub3
Pharmacotherapy for hypertension-induced left ventricular hypertrophy
Abstract
Background: Hypertension is the leading preventable risk factor for cardiovascular disease and premature death worldwide. One of the clinical effects of hypertension is left ventricular hypertrophy (LVH), a process of cardiac remodelling. It is estimated that over 30% of people with hypertension also suffer from LVH, although the prevalence rates vary according to the LVH diagnostic criteria. Severity of LVH is associated with a higher prevalence of cardiovascular disease and an increased risk of death. The role of antihypertensives in the regression of left ventricular mass has been extensively studied. However, uncertainty exists regarding the role of antihypertensive therapy compared to placebo in the morbidity and mortality of individuals with hypertension-induced LVH.
Objectives: To assess the effect of antihypertensive pharmacotherapy compared to placebo or no treatment on morbidity and mortality of adults with hypertension-induced LVH.
Search methods: Cochrane Hypertension's Information Specialist searched the following databases for studies: Cochrane Hypertension Specialised Register (to 26 September 2020), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library; 2020, Issue 9), Ovid MEDLINE (1946 to 22 September 2020), and Ovid Embase (1974 to 22 September 2020). We searched the World Health Organization International Clinical Trials Registry Platform and the ClinicalTrials.gov for ongoing trials. We also searched Epistemonikos (to 19 February 2021), LILACS BIREME (to 19 February 2021), and Clarivate Web of Science (to 26 February 2021), and contacted authors and funders of the identified trials to obtain additional information and individual participant data. There were no language restrictions.
Selection criteria: Randomised controlled trials (RCTs) with at least 12 months' follow-up comparing antihypertensive pharmacological therapy (monotherapy or in combination) with placebo or no treatment in adults (18 years of age or older) with hypertension-induced LVH were eligible for inclusion. The trials must have analysed at least one primary outcome (all-cause mortality, cardiovascular events, or total serious adverse events) to be considered for inclusion.
Data collection and analysis: Two review authors screened the search results, with any disagreements resolved by consensus amongst all review authors. Two review authors carried out the data extraction and analyses. We assessed risk of bias of the included studies following Cochrane methodology. We used the GRADE approach to assess the certainty of the body of evidence.
Main results: We included three multicentre RCTs. We selected 930 participants from the included studies for the analyses, with a mean follow-up of 3.8 years (range 3.5 to 4.3 years). All of the included trials performed an intention-to-treat analysis. We obtained evidence for the review by identifying the population of interest from the trials' total samples. None of the trials provided information on the cause of LVH. The intervention varied amongst the included trials: hydrochlorothiazide plus triamterene with the possibility of adding alpha methyldopa, spironolactone, or olmesartan. Placebo was administered to participants in the control arm in two trials, whereas participants in the control arm of the remaining trial did not receive any add-on treatment. The evidence is very uncertain regarding the effect of additional antihypertensive pharmacological therapy compared to placebo or no treatment on mortality (14.3% intervention versus 13.6% control; risk ratio (RR) 1.02, 95% confidence interval (CI) 0.74 to 1.40; 3 studies; 930 participants; very low-certainty evidence); cardiovascular events (12.6% intervention versus 11.5% control; RR 1.09, 95% CI 0.77 to 1.55; 3 studies; 930 participants; very low-certainty evidence); and hospitalisation for heart failure (10.7% intervention versus 12.5% control; RR 0.82, 95% CI 0.57 to 1.17; 2 studies; 915 participants; very low-certainty evidence). Although both arms yielded similar results for total serious adverse events (48.9% intervention versus 48.1% control; RR 1.02, 95% CI 0.89 to 1.16; 3 studies; 930 participants; very low-certainty evidence) and total adverse events (68.3% intervention versus 67.2% control; RR 1.07, 95% CI 0.86 to 1.34; 2 studies; 915 participants), the incidence of withdrawal due to adverse events may be significantly higher with antihypertensive drug therapy (15.2% intervention versus 4.9% control; RR 3.09, 95% CI 1.69 to 5.66; 1 study; 522 participants; very low-certainty evidence). Sensitivity analyses limited to blinded trials, trials with low risk of bias in core domains, and trials with no funding from the pharmaceutical industry did not change the results of the main analyses. Limited evidence on the change in left ventricular mass index prevented us from drawing any firm conclusions.
Authors' conclusions: We are uncertain about the effects of adding additional antihypertensive drug therapy on the morbidity and mortality of participants with LVH and hypertension compared to placebo. Although the incidence of serious adverse events was similar between study arms, additional antihypertensive therapy may be associated with more withdrawals due to adverse events. Limited and low-certainty evidence requires that caution be used when interpreting the findings. High-quality clinical trials addressing the effect of antihypertensives on clinically relevant variables and carried out specifically in individuals with hypertension-induced LVH are warranted.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Leire Leache has no conflicts to declare.
Marta Gutiérrez‐Valencia has no conflicts to declare.
Rosa M Finizola has no conflicts to declare.
Elizabeth Infante has no conflicts to declare.
Bartolome Finizola has no conflicts to declare.
Jordi Pardo Pardo has no conflicts to declare.
Yris Flores has no conflicts to declare.
Ricardo Granero has no conflicts to declare.
Kaduo J Arai has no conflicts to declare.
Figures
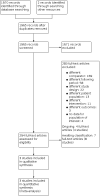
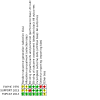










Update of
- doi: 10.1002/14651858.CD012039.pub2
References
References to studies included in this review
EWPHE 1991 {published and unpublished data}
-
- Amery A, Berthaux P, Birkenhäger W, Boel A, Brixko P, Bulpitt C, et al. Antihypertensive therapy in patients above age 60 years (Fourth Interim report of the European Working Party on High Blood pressure in Elderly: EWPHE). Clinical Science and Molecular Medicine. Supplement 1978;4:163s-270s. [DOI: 10.1042/cs055263s] - DOI - PubMed
-
- Amery A, Birkenhäger W, Brixko P, Bulpitt C, Clement D, Leeuw P, et al. Influence of hypotensive drug treatment in elderly hypertensives: study terminating events in the trial of the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension. Supplement 1985;3(3):S501-11. [PMID: ] - PubMed
-
- Amery A, Birkenhäger W, Brixko R, Bulpitt C, Clement D, Deruyttere M, et al. Efficacy of antihypertensive drug treatment according to age, sex, blood pressure, and previous cardiovascular disease in patients over the age of 60. Lancet 1986;2(8507):589-92. [DOI: 10.1016/s0140-6736(86)92424-4] - DOI - PubMed
-
- Amery A, Birkenhäger W, Bulpitt C, Clement D, Leeuw P, Deruyttere ML, et al. Diuretics - a risk in the long-term treatment of hypertensive patients? Journal of Hypertension 1988;6(11):125-30. [PMID: ] - PubMed
SUPPORT 2015 {published and unpublished data}
-
- Sakata Y, Nochioka K, Miura M, Takada T, Tadaki S, Miyata S, et al. Supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial - rationale and design. Journal of Cardiology 2013;62(1):31-6. [DOI: 10.1016/j.jjcc.2013.02.011] - DOI - PubMed
-
- Sakata Y, Shiba N, Takahashi J, Miyata S, Nochioka K, Miura M, et al. Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: the supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial. European Heart Journal 2015;36(15):915-23. [DOI: 10.1093/eurheartj/ehu504] - DOI - PMC - PubMed
TOPCAT 2014 {published and unpublished data}
-
- Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. American Heart Journal 2011;162(6):966-72. [DOI: 10.1016/j.ahj.2011.09.007] - DOI - PubMed
-
- Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131(1):34-42. [DOI: 10.1161/CIRCULATIONAHA.114.013255] - DOI - PubMed
-
- Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circulation. Heart Failure 2014;7(5):740-51. [DOI: 10.1161/CIRCHEARTFAILURE.114.001583] - DOI - PMC - PubMed
-
- Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, et al, TOPCAT Investigators. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circulation. Heart Failure 2014;7(1):104-15. [DOI: 10.1161/CIRCHEARTFAILURE.113.000887] - DOI - PMC - PubMed
References to studies excluded from this review
Black 2001 {published data only}
-
- Black HR, Elliott WJ, Weber MA, Frishman WH, Strom JA, Liebson PR, et al. One-year study of felodipine or placebo for stage 1 isolated systolic hypertension. Hypertension 2001;38(5):1118-23. [DOI: 10.1161/hy1101.095000. PMID: 11711508] - PubMed
Hernández 2000 {published data only}
HOPE 2003 {published data only}
-
- Lonn E, Mathew J, Pogue J, Johnstone D, Danisa K, Bosch J, et al. Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high-risk patients. European Journal of Cardiovascular Prevention and Rehabilitation 2003;16(6):420-8. [DOI: 10.1097/01.hjr.0000106836.97722.cf] - DOI - PubMed
RENAAL 2005 {published data only}
-
- Boner G, Cooper ME, McCarroll K, Brenner BM, Zeeuw D, Kowey PR, et al. RENAAL Investigators. Adverse effects of left ventricular hypertrophy in the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) study. Diabetologia 2005;48(10):1980-7. [DOI: 10.1007/s00125-005-1893-1] - DOI - PubMed
TCCGIH 1994 {published data only}
-
- The Chengdu Collaborative Group on Intervention trial of Hypertension (TCCGIH). Nifedipine intervention trial of hypertension - a randomized, placebo controlled study. Chinese Journal of Cardiology 1994;22(3):201-5.
VALIDD 2007 {published data only}
-
- Janardhanan R, Daley W, Zile M, Aurigemma G, Naqvi TZ, Lacourcière Y, et al. Abstract 2543: Left ventricular diastolic function in a broad range of patients with hypertension: the Valsartan in Diastolic Dysfunction (VALIDD) Trial. Circulation 2006;114(Suppl 18):II_525. [www.ahajournals.org/doi/10.1161/circ.114.suppl_18.II_525-c]
-
- Janardhanan R, Daley WL, Naqvi TZ, Mulvagh SL, Aurigemma G, Zile M, et al. Rationale and design: the VALsartan In Diastolic Dysfunction (VALIDD) Trial: evolving the management of diastolic dysfunction in hypertension. American Heart Journal 2006;152(2):246-52. [DOI: 10.1016/j.ahj.2006.01.009] - DOI - PubMed
-
- Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet 2007;369(9579):2079-87. [DOI: 10.1016/S0140-6736(07)60980-5] - DOI - PubMed
References to studies awaiting assessment
CHARM 1999 {published data only}
-
- Hawkins NM, Wang D, McMurray JJ, Pfeffer MA, Swedberg K, Granger CB, et al, CHARM Investigators and Committees. Prevalence and prognostic implications of electrocardiographic left ventricular hypertrophy in heart failure: evidence from the CHARM programme. Heart 2007;93(1):59-64. [DOI: 10.1136/hrt.2005.083949] - DOI - PMC - PubMed
-
- Swedberg K, Pfeffer M, Granger C, Held P, McMurray J, Ohlin G, et al Charm-Programme Investigators. Candesartan in heart failure - assessment of reduction in mortality and morbidity (CHARM): rationale and design. Journal of Cardiac Failure 1999;5(3):276-82. [DOI: 10.1016/s1071-9164(99)90013-1] - DOI - PubMed
FEVER 2005 {published data only}
-
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. Journal of Hypertension 2005;23(12):2157-72. [DOI: 10.1097/01.hjh.0000194120.42722.ac] - DOI - PubMed
HYVET 2001 {published data only}
-
- Antikainen RL, Peters R, Beckett NS, Fagard RH, Wang JG, Rajkumar C, et al. Left ventricular hypertrophy is a predictor of cardiovascular events in elderly hypertensive patients: Hypertension in the Very Elderly Trial. Journal of Hypertension 2016;34(11):2280-6. [DOI: 10.1097/HJH.0000000000001073] - DOI - PubMed
PROFESS 2007 {published data only}
-
- Diener HC, Sacco R, Yusuf S. Rationale, design and baseline data of a randomized, double-blind, controlled trial comparing two antithrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: the Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS). Cerebrovascular Diseases 2007;23(5-6):368-80. [DOI: 10.1159/000100105] - DOI - PubMed
RALES 1999 {published data only}
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, et al, Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. New England Journal of Medicine 1999;341(10):709-17. [DOI: 10.1056/NEJM199909023411001] - DOI - PubMed
Syst‐Eur 1991 {published data only}
-
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al, The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350(9080):757-64. [DOI: 10.1016/s0140-6736(97)05381-6] - DOI - PubMed
TRANSCEND 2004 {published data only}
-
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372(9644):1174-83. [DOI: 10.1016/S0140-6736(08)61242-8] - DOI - PubMed
-
- Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. American Heart Journal 2004;148(1):52-61. [DOI: 10.1016/j.ahj.2004.03.020] - DOI - PubMed
VAL‐HeFT 2001 {published data only}
-
- Florea VG, Rector TS, Anand IS, Cohn JN. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the Valsartan Heart Failure Trial. Circulation. Heart Failure 2016;9(7):e003123. [DOI: 10.1161/CIRCHEARTFAILURE.116.003123] - DOI - PubMed
References to ongoing studies
ChiCTR‐INR‐16008079 {published data only}
-
- ChiCTR-INR-16008079. Aldosterone antagonist delays the progression of heart failure with preserved ejection fraction: a randomised controlled clinical trial. www.chictr.org.cn/showprojen.aspx?proj=13650.
ChiCTR‐IPR‐16009507 {published data only}
-
- ChiCTR-IPR-16009507. Aldosterone antagonist delays the progression of diastolic dysfunction in patients with hypertension and myocardial hypertrophy: a randomised controlled clinical trial. www.chictr.org.cn/showprojen.aspx?proj=16311.
NCT02893358 {published data only}
-
- NCT02893358. Antihypertensive Treatment in Masked Hypertension for Target Organ Protection (ANTI-MASK). www.clinicaltrials.gov/ct2/show/NCT02893358.
NCT03315832 {published data only}
-
- NCT03315832. Efficacy of Angiotensin Receptor Blocker Following aortIc Valve Intervention for Aortic STenOsis: a Randomized mulTi-cEntric Double-blind Phase II Study (ARISTOTE). www.clinicaltrials.gov/ct2/show/NCT03315832.
Additional references
ACC‐AHA 2017
-
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Journal of the American College of Cardiology 2017;17:S0735–1097. [DOI: 10.1016/j.jacc.2017.11.006] - DOI
Adams 1997
-
- Adams RD, Victor M, Ropper AH. Principles of Neurology. 6th edition. Columbus, OH: McGraw-Hill, 1997.
Águila‐Marín 2013
-
- Águila-Marín J. Ventricular hypertrophy. Part I [Hipertrofia ventricular izquierda. Parte I]. Revista de Medicina e Investigación 2013;1(1):25-30.
Ang 2008
Aronow 2017
Balshem 2011
Bauml 2010
Brok 2008
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive. Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] - DOI - PubMed
Cohn 2000
-
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling - concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. Journal of the American College of Cardiology 2000;35(3):569-82. [DOI: 10.1016/s0735-1097(99)00630-0] - DOI - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Version accessed 26 April 2021. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org.
CTU 2011 [Computer program]
-
- Centre for Clinical Intervention Research, Trial Unit TSA - Trial Sequential Analysis. Copenhagen: Centre for Clinical Intervention Research, Trial Unit, 2011.
Cuspidi 2012
Cuspidi 2019a
Cuspidi 2019b
Devereux 2004
Dweck 2012
-
- Dweck MR, Joshi S, Murigu T, Gulati A, Alpendurada F, Jabbour A, et al. Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance 2012;14(1):50. [DOI: 10.1186/1532-429X-14-50] - DOI - PMC - PubMed
ESC/ESH 2018
-
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). European Heart Journal 2018;39(33):3021–104. [DOI: 10.1093/eurheartj/ehy339] - DOI - PubMed
Fagard 2009
Fisch 1993
-
- Fisch C. Tratado de Cardiología. Medicina Cardiovascular. 4th edition. Madrid: McGraw-Hill Interamericana de España, 1993.
GBD 2017
-
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923-94. [DOI: 10.1016/S0140-6736(18)32225-6] - DOI - PMC - PubMed
Giuliani 1996
-
- Giuliani ER, Gersh BJ, McGoon MD, Hayes DL, Schaff HV. Mayo Clinic Practice of Cardiology. 3rd edition. St Louis, MO: Mosby, 1996.
González‐Juanatey 2007
-
- González-Juanatey J, Cea-Calvo L, Bertomeu V, Aznar J. Electrocardiographic criteria for left ventricular hypertrophy and cardiovascular risk in hypertensives. VIIDA study [Criterios electrocardiográficos de hipertrofia ventricular izquierda y perfil de riesgo cardiovascular en hipertensos. Estudio VIIDA]. Revista Española de Cardiología 2007;60(2):148-56. [PMID: ] - PubMed
Gradman 2006
Guyatt 2011a
Guyatt 2011b
Guyatt 2011c
Guyatt 2011d
Guyatt 2011e
Guyatt 2011f
Guyatt 2011g
Guyatt 2011h
Guyatt 2011i
Guyatt 2013
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151-7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Hernández 2000
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. - PMC - PubMed
Hypertension Canada 2020
-
- Rabi DM, McBrien KA, Sapir-Pichhadze R, Nakhla M, Ahmed SB, Dumanski SM, et al. Hypertension Canada's 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children. Canadian Journal of Cardiology 2020;36(5):596-624. [DOI: 10.1016/j.cjca.2020.02.086] - DOI - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice 1997 CFR & ICH Guidelines. Geneva: Barnett International/PAREXEL, 1997.
ISH 2020
Khouri 2010
Klingbeil 2003
Lang 2005
-
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography 2005;18(12):1440-63. [DOI: 10.1016/j.echo.2005.10.005] - DOI - PubMed
Lewington 2002
-
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903-13. [DOI: 10.1016/s0140-6736(02)11911-8] - DOI - PubMed
Llancaqueo 2012
-
- Llancaqueo M. Left ventricular hypertrophy and cardiovascular risk factor in hypertensive patients [Hipertrofia ventricular izquierda como factor de riesgo cardiovascular en el paciente hipertenso]. Revista Médica Clínica Las Condes 2012;23(6):707-14.
Lorell 2000
Malmqvist 2001
-
- Malmqvist K, Kahan T, Eriksson S, Björkander I, Held C, Forslund L, et al. Evaluation of various electrocardiographic criteria for left ventricular hypertrophy in patients with stable angina pectoris: influence of using modified limb electrodes. Clinical Physiology 2001;21(2):196-207. [DOI: 10.1046/j.1365-2281.2001.00310.x] - DOI - PubMed
Mills 2016
Mills 2020
NCD‐RisC 2017
NICE 2019
-
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. www.nice.uk/guidance/ng136 (accessed 2 December 2020).
Oktay 2016
Pérez de la Isla 2010
-
- Pérez de la Isla L, Saltijeral A. Echo transducer and decision making in hypertension [El transductor de eco y toma de decisiones en hipertensión arterial]. Avances Cardiológicos 2010;30(4):388-92.
Pierdomenico 2010
Raman 2010
Rapsomaniki 2014
-
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014;383(9932):1899-911. [DOI: 10.1016/S0140-6736(14)60685-1] - DOI - PMC - PubMed
RevMan Web 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 3.7.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Romhilt 1968
Romhilt 1969
Roush 2018
-
- Roush GC, Abdelfattah R, Song S, Kostis JB, Ernst ME, Sica DA. Hydrochlorothiazide and alternative diuretics versus renin-angiotensin system inhibitors for the regression of left ventricular hypertrophy: a head-to-head meta-analysis. Journal of Hypertension 2018;36(6):1247-55. [DOI: 10.1097/HJH.0000000000001691] - DOI - PubMed
Schmieder 2000
Soliman 2017
Sterne 2011
Thorlund 2009
Thorlund 2010
-
- Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. Clinical Epidemiology 2010;2:57-66. [DOI: 10.2147/clep.s9242] - DOI - PMC - PubMed
Thorlund 2017
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). ctu.dk/tsa/ (accessed 10 May 2021).
Verdecchia 2004
Wetterslev 2008
Wetterslev 2009
WHO 2019
-
- World Health Organization Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2019. www.whocc.no/atc_ddd_index/ (accessed 5 July 2019).
Xing 2018
Yang 2013
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

