[Recommendations for use of the diverse tests for detection of SARS-COV-2 infection]
- PMID: 34629849
- PMCID: PMC7556773
- DOI: 10.1016/j.enfcli.2020.10.001
[Recommendations for use of the diverse tests for detection of SARS-COV-2 infection]
Abstract
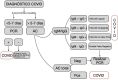
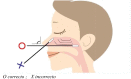
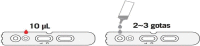
In the current crisis caused by SARS-CoV-2, there is a global need to know and combat the virus. One of the strategies is to track and diagnose cases in order to isolate and interrupt the epidemiological chain. Therefore, the aim of this article is to describe the different most used diagnostic tests and analyze their validity and indications for use according to scientific evidence and the main recommendations of scientific societies and reference organizations at national and international level. Since the beginning of the pandemic, the availability of tests has been subject to the conditions of the manufacturing market itself and to the guidelines set in each country. Among the most used types of tests, it is worth highlighting PCR, antibody detection tests (IgG and IGM) and total antibodies (Ab), also known as rapid tests, and tests for the detection of antigens in nasopharyngeal exudate or other upper/lower respiratory samples. For each of these tests, it is necessary to know their recommendations for use and the procedure for taking samples, which is essential to minimize alterations in the results due to poor handling. Likewise, it is necessary to determine the most appropriate moment for taking samples and their adequate interpretation of the results obtained, which must always be considered together with the patient's symptoms for clinical decision-making.
En la actual crisis provocada por el SARS-CoV-2, surge la necesidad global de conocer y combatir el virus. Una de las estrategias es rastrear y diagnosticar los casos con el fin de aislar e interrumpir la cadena epidemiológica. Por ello, el objetivo de este artículo es describir las distintas pruebas diagnósticas más utilizadas y analizar su validez e indicaciones de uso según la evidencia científica y las principales recomendaciones de sociedades científicas y organismos de referencia nacionales e internacionales. Desde el inicio de la pandemia, la disponibilidad de test ha estado supeditada a las condiciones del propio mercado de fabricación y a las directrices marcadas en cada país. Entre los tipos de test más utilizados cabe destacar la PCR, los test de detección de anticuerpos (IgG e IGM) y anticuerpos totales (Ab), también conocidos como test rápidos, y los test de detección de antígenos en exudado nasofaríngeo u otras muestras respiratorias de vías altas/bajas. Para cada uno de estos test es necesario conocer sus recomendaciones de uso y el procedimiento para la toma de muestras, siendo fundamental para minimizar las alteraciones en los resultados debidas a una manipulación deficitaria. Asimismo, es necesario determinar el momento más adecuado para la toma de muestras y la adecuada interpretación de los resultados obtenidos, que siempre ha de ser considerada junto con la sintomatología del paciente para la toma de decisiones clínicas.
Keywords: COVID-19; Coronavirus infections; Diagnosis; SARS virus; SARS-CoV-2; Test.
© 2020 Published by Elsevier España, S.L.U.
Figures
References
-
- Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nat Biotechnol [Internet]. 2020;38:515–518. [consultado 5 May 2020]. Disponible en: http://www.nature.com/articles/d41587-020-00010-2. - PubMed
-
- Korean Society of Infectious Diseases, Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-associated Infection Control and Prevention, and Korea Centers for Disease Control and Prevention Report on the epidemiological features of Coronavirus Disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci [Internet]. 2020;35 [consultado 10 May 2020]. Disponible en: https://jkms.org/DOIx.php?id=10.3346/jkms.2020.35.e112. - PMC - PubMed
-
- Central Disease Control Headquarters. COVID-19 Response. Coronavirus Disease-19, Republic of Korea Korean Government's Response System (as of February 25, 2020) [Internet] [consultado 5 May 2020]. Disponible en: http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=111&dataGubun=&n...=
-
- Cheng M.P., Lee T.C., Tan D.H.S., Murthy S. Generating randomized trial evidence to optimize treatment in the COVID-19 pandemic. Can Med Assoc J [Internet]. 2020;192:E405–E407. Disponible en: http://www.cmaj.ca/lookup/doi/10.1503/cmaj.200438. - DOI - PMC - PubMed
-
- Cheng M.P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M. Diagnostic Testing for Severe Acute Respiratory Syndrome–Related Coronavirus-2. Ann Intern Med [Internet]. 2020;13:M20–M1301. Disponible en: https://www.acpjournals.org/doi/10.7326/M20-1301. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

