Conditional Deletion of EphA4 on Cx3cr1-Expressing Microglia Fails to Influence Histopathological Outcome and Blood Brain Barrier Disruption Following Brain Injury
- PMID: 34630039
- PMCID: PMC8497746
- DOI: 10.3389/fnmol.2021.747770
Conditional Deletion of EphA4 on Cx3cr1-Expressing Microglia Fails to Influence Histopathological Outcome and Blood Brain Barrier Disruption Following Brain Injury
Abstract
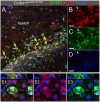
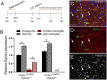
Erythropoietin-producing human hepatocellular receptors play a major role in central nervous system injury. Preclinical and clinical studies revealed the upregulation of erythropoietin-producing human hepatocellular A4 (EphA4) receptors in the brain after acute traumatic brain injury. We have previously reported that Cx3cr1-expressing cells in the peri-lesion show high levels of EphA4 after the induction of controlled cortical impact (CCI) injury in mice. Cx3cr1 is a fractalkine receptor expressed on both resident microglia and peripheral-derived macrophages. The current study aimed to determine the role of microglial-specific EphA4 in CCI-induced damage. We used Cx3cr1 CreER/+ knock-in/knock-out mice, which express EYFP in Cx3cr1-positive cells to establish microglia, EphA4-deficient mice following 1-month tamoxifen injection. Consistent with our previous findings, induction of CCI in wild-type (WT) Cx3cr1 CreER/+ EphA4 +/+ mice increased EphA4 expression on EYFP-positive cells in the peri-lesion. To distinguish between peripheral-derived macrophages and resident microglia, we exploited GFP bone marrow-chimeric mice and found that CCI injury increased EphA4 expression in microglia (TMEM119+GFP-) using immunohistochemistry. Using Cx3cr1 CreER/+ EphA4 f/f (KO) mice, we observed that the EphA4 mRNA transcript was undetected in microglia but remained present in whole blood when compared to WT. Finally, we found no difference in lesion volume or blood-brain barrier (BBB) disruption between WT and KO mice at 3 dpi. Our data demonstrate a nonessential role of microglial EphA4 in the acute histopathological outcome in response to CCI.
Keywords: Eph signaling; TMEM119; innate immune; neuroinflammation; peripheral monocytes; traumatic brain injury.
Copyright © 2021 Soliman, Mills, Ju, Kaloss, Basso, Groot, Kelly, Kowalski, Elhassanny, Chen, Wang and Theus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Efferocytosis is restricted by axon guidance molecule EphA4 via ERK/Stat6/MERTK signaling following brain injury.J Neuroinflammation. 2023 Nov 9;20(1):256. doi: 10.1186/s12974-023-02940-5. J Neuroinflammation. 2023. PMID: 37941008 Free PMC article.
-
Peripheral loss of EphA4 ameliorates TBI-induced neuroinflammation and tissue damage.J Neuroinflammation. 2019 Nov 11;16(1):210. doi: 10.1186/s12974-019-1605-2. J Neuroinflammation. 2019. PMID: 31711546 Free PMC article.
-
An overlooked subset of Cx3cr1wt/wt microglia in the Cx3cr1CreER-Eyfp/wt mouse has a repopulation advantage over Cx3cr1CreER-Eyfp/wt microglia following microglial depletion.J Neuroinflammation. 2022 Jan 21;19(1):20. doi: 10.1186/s12974-022-02381-6. J Neuroinflammation. 2022. PMID: 35062962 Free PMC article.
-
Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
Atypical Neurogenesis, Astrogliosis, and Excessive Hilar Interneuron Loss Are Associated with the Development of Post-Traumatic Epilepsy.Cells. 2023 Apr 25;12(9):1248. doi: 10.3390/cells12091248. Cells. 2023. PMID: 37174647 Free PMC article.
-
Endothelial deletion of EPH receptor A4 alters single-cell profile and Tie2/Akap12 signaling to preserve blood-brain barrier integrity.Proc Natl Acad Sci U S A. 2023 Oct 10;120(41):e2204700120. doi: 10.1073/pnas.2204700120. Epub 2023 Oct 5. Proc Natl Acad Sci U S A. 2023. PMID: 37796990 Free PMC article.
-
Skull bone marrow-derived immune cells infiltrate the injured cerebral cortex and exhibit anti-inflammatory properties.Brain Behav Immun. 2025 Jan;123:244-253. doi: 10.1016/j.bbi.2024.09.023. Epub 2024 Sep 16. Brain Behav Immun. 2025. PMID: 39293691
-
Unraveling the Potential of EphA4: A Breakthrough Target and Beacon of Hope for Neurological Diseases.Cell Mol Neurobiol. 2023 Oct;43(7):3375-3391. doi: 10.1007/s10571-023-01390-0. Epub 2023 Jul 21. Cell Mol Neurobiol. 2023. PMID: 37477786 Free PMC article. Review.
-
Efferocytosis is restricted by axon guidance molecule EphA4 via ERK/Stat6/MERTK signaling following brain injury.J Neuroinflammation. 2023 Nov 9;20(1):256. doi: 10.1186/s12974-023-02940-5. J Neuroinflammation. 2023. PMID: 37941008 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

