Sirtuins as Potential Therapeutic Targets for Mitigating Neuroinflammation Associated With Alzheimer's Disease
- PMID: 34630044
- PMCID: PMC8492950
- DOI: 10.3389/fncel.2021.746631
Sirtuins as Potential Therapeutic Targets for Mitigating Neuroinflammation Associated With Alzheimer's Disease
Abstract
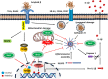
Alzheimer's disease (AD) is the most common neurodegenerative disorder, which is associated with memory deficit and global cognitive decline. Age is the greatest risk factor for AD and, in recent years, it is becoming increasingly appreciated that aging-related neuroinflammation plays a key role in the pathogenesis of AD. The presence of β-amyloid plaques and neurofibrillary tangles are the primary pathological hallmarks of AD; defects which can then activate a cascade of molecular inflammatory pathways in glial cells. Microglia, the resident macrophages in the central nervous system (CNS), are the major triggers of inflammation; a response which is typically intended to prevent further damage to the CNS. However, persistent microglial activation (i.e., neuroinflammation) is toxic to both neurons and glia, which then leads to neurodegeneration. Growing evidence supports a central role for sirtuins in the regulation of neuroinflammation. Sirtuins are NAD+-dependent protein deacetylases that modulate a number of cellular processes associated with inflammation. This review examines the latest findings regarding AD-associated neuroinflammation, mainly focusing on the connections among the microglial molecular pathways of inflammation. Furthermore, we highlight the biology of sirtuins, and their role in neuroinflammation. Suppression of microglial activity through modulation of the sirtuin activity has now become a key area of research, where progress in therapeutic interventions may slow the progression of Alzheimer's disease.
Keywords: Alzheimer’s disease-AD; NF-kB; NLRP3 inflammasome; microglia; neuroinflammantion; sirtuin activators; sirtuins (SIRT).
Copyright © 2021 Fernando and Wijayasinghe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., et al. (2009). Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183 787–791. 10.4049/jimmunol.0901363 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

