Sensory-Evoked 40-Hz Gamma Oscillation Improves Sleep and Daily Living Activities in Alzheimer's Disease Patients
- PMID: 34630050
- PMCID: PMC8500065
- DOI: 10.3389/fnsys.2021.746859
Sensory-Evoked 40-Hz Gamma Oscillation Improves Sleep and Daily Living Activities in Alzheimer's Disease Patients
Abstract
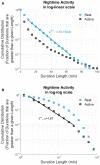
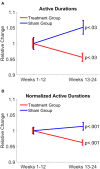
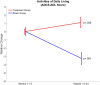
Pathological proteins contributing to Alzheimer's disease (AD) are known to disrupt normal neuronal functions in the brain, leading to unbalanced neuronal excitatory-inhibitory tone, distorted neuronal synchrony, and network oscillations. However, it has been proposed that abnormalities in neuronal activity directly contribute to the pathogenesis of the disease, and in fact it has been demonstrated that induction of synchronized 40 Hz gamma oscillation of neuronal networks by sensory stimulation reverses AD-related pathological markers in transgenic mice carrying AD-related human pathological genes. Based on these findings, the current study evaluated whether non-invasive sensory stimulation inducing cortical 40 Hz gamma oscillation is clinically beneficial for AD patients. Patients with mild to moderate AD (n = 22) were randomized to active treatment group (n = 14; gamma sensory stimulation therapy) or to sham group (n = 8). Participants in the active treatment group received precisely timed, 40 Hz visual and auditory stimulations during eye-closed condition to induce cortical 40 Hz steady-state oscillations in 1-h daily sessions over a 6-month period. Participants in the sham group were exposed to similar sensory stimulation designed to not evoke cortical 40 Hz steady-state oscillations that are observed in the active treatment patients. During the trial, nighttime activities of the patients were monitored with continuous actigraphy recordings, and their functional abilities were measured by Alzheimer's Disease Cooperative Study - Activities of Daily Living (ADCS-ADL) scale. Results of this study demonstrated that 1-h daily therapy was well tolerated throughout the 6-month treatment period by all subjects. Patients receiving gamma sensory stimulation showed significantly reduced nighttime active periods, in contrast, to deterioration in sleep quality in sham group patients. Patients in the sham group also showed the expected, significant decline in ADCS-ADL scores, whereas patients in the gamma sensory stimulation group fully maintained their functional abilities over the 6-month period. These findings confirm the safe application of 40 Hz sensory stimulation in AD patients and demonstrate a high adherence to daily treatment. Furthermore, this is the first time that beneficial clinical effects of the therapy are reported, justifying expanded and longer trials to explore additional clinical benefits and disease-modifying properties of gamma sensory stimulation therapy. Clinical Trial Registration: clinicaltrials.gov, identifier: NCT03556280.
Keywords: Alzheimer’s disease; actigraphy; activities of daily living; sensory-evoked gamma oscillation; sleep.
Copyright © 2021 Cimenser, Hempel, Travers, Strozewski, Martin, Malchano and Hajós.
Conflict of interest statement
All authors were employed by Cognito Therapeutics, Inc. at the time of this study.
Figures

References
-
- Ancoli-Israel S., Palmer B. W., Cooke J. R., Corey-Bloom J., Fiorentino L., Natarajan L., et al. (2008). Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J. Am. Geriatr. Soc. 56 2076–2081. 10.1111/j.1532-5415.2008.01934.x - DOI - PMC - PubMed
-
- Bubu O. M., Andrade A. G., Umasabor-Bubu O. Q., Hogan M. M., Turner A. D., de Leon M. J., et al. (2020). Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med. Rev. 50:101250. 10.1016/j.smrv.2019.101250 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

