Measuring Behavior in the Home Cage: Study Design, Applications, Challenges, and Perspectives
- PMID: 34630052
- PMCID: PMC8498589
- DOI: 10.3389/fnbeh.2021.735387
Measuring Behavior in the Home Cage: Study Design, Applications, Challenges, and Perspectives
Abstract
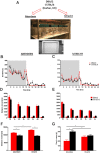
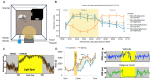
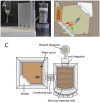
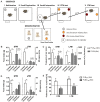
The reproducibility crisis (or replication crisis) in biomedical research is a particularly existential and under-addressed issue in the field of behavioral neuroscience, where, in spite of efforts to standardize testing and assay protocols, several known and unknown sources of confounding environmental factors add to variance. Human interference is a major contributor to variability both within and across laboratories, as well as novelty-induced anxiety. Attempts to reduce human interference and to measure more "natural" behaviors in subjects has led to the development of automated home-cage monitoring systems. These systems enable prolonged and longitudinal recordings, and provide large continuous measures of spontaneous behavior that can be analyzed across multiple time scales. In this review, a diverse team of neuroscientists and product developers share their experiences using such an automated monitoring system that combines Noldus PhenoTyper® home-cages and the video-based tracking software, EthoVision® XT, to extract digital biomarkers of motor, emotional, social and cognitive behavior. After presenting our working definition of a "home-cage", we compare home-cage testing with more conventional out-of-cage tests (e.g., the open field) and outline the various advantages of the former, including opportunities for within-subject analyses and assessments of circadian and ultradian activity. Next, we address technical issues pertaining to the acquisition of behavioral data, such as the fine-tuning of the tracking software and the potential for integration with biotelemetry and optogenetics. Finally, we provide guidance on which behavioral measures to emphasize, how to filter, segment, and analyze behavior, and how to use analysis scripts. We summarize how the PhenoTyper has applications to study neuropharmacology as well as animal models of neurodegenerative and neuropsychiatric illness. Looking forward, we examine current challenges and the impact of new developments. Examples include the automated recognition of specific behaviors, unambiguous tracking of individuals in a social context, the development of more animal-centered measures of behavior and ways of dealing with large datasets. Together, we advocate that by embracing standardized home-cage monitoring platforms like the PhenoTyper, we are poised to directly assess issues pertaining to reproducibility, and more importantly, measure features of rodent behavior under more ethologically relevant scenarios.
Keywords: EthoVision XT; PhenoTyper; home-cage; neuroscience; rodent behavior; video-tracking.
Copyright © 2021 Grieco, Bernstein, Biemans, Bikovski, Burnett, Cushman, van Dam, Fry, Richmond-Hacham, Homberg, Kas, Kessels, Koopmans, Krashes, Krishnan, Logan, Loos, McCann, Parduzi, Pick, Prevot, Riedel, Robinson, Sadighi, Smit, Sonntag, Roelofs, Tegelenbosch and Noldus.
Conflict of interest statement
BB and QP were employed by F. Hoffman-La Roche Ltd. BK and ML are employed by Synaptologics BV. ED, FG, LN, RFR, and RT are employed by Noldus Information Technology BV. AS participates in a holding that owns shares of Synaptologics BV. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Archer J. (1976). “The organisation of aggression and fear in vertebrates,” in Perspectives in Ethology (Vol. 2), eds Bateson P. P. G., Klopfer P. H. (Boston, MA: Springer; ), 231–298.
Grants and funding
LinkOut - more resources
Full Text Sources

