Screening for Susceptibility-Related Biomarkers of Diclofenac-Induced Liver Injury in Rats Using Metabolomics
- PMID: 34630079
- PMCID: PMC8494976
- DOI: 10.3389/fphar.2021.693928
Screening for Susceptibility-Related Biomarkers of Diclofenac-Induced Liver Injury in Rats Using Metabolomics
Abstract
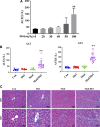
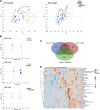
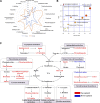
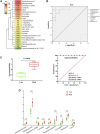
Early identification of individuals susceptible to idiosyncratic drug-induced liver injury (IDILI) is a challenging unmet demand. Diclofenac, one of the most widely available over-the-counter drugs for pain management worldwide, may induce liver dysfunction, acute liver failure, and death. Herein, we report that diclofenac-related hepatobiliary adverse reactions occurred more frequently in cases with immune activation. Furthermore, experiments with rats demonstrated divergent hepatotoxicity responses in individuals exposed to diclofenac, and modest inflammation potentiated diclofenac-induced liver injury. Susceptible rats had unique plasma metabolomic characteristics, and as such, the metabolomic approach could be used to distinguish susceptible individuals. The 23 identified susceptibility-related metabolites were enriched by several metabolic pathways related to acute-phase reactions of immunocytes and inflammatory responses, including sphingolipid, tyrosine, phenylalanine, tryptophan, and lipid metabolism pathways. This finding implies a mechanistic role of metabolic and immune disturbances affects susceptibility to diclofenac-IDILI. Further nine metabolite biomarkers with potent diagnostic capabilities were identified using receiver operating characteristic curves. These findings elucidated the potential utility of metabolomic biomarkers to identify individuals susceptible to drug hepatotoxicity and the underlying mechanism of metabolic and immune disturbances occurring in IDILI.
Keywords: biomarker; diclofenac; idiosyncratic drug-induced liver injury; metabolomics; susceptible individual.
Copyright © 2021 Tu, Gao, Song, Niu, Ma, Zhou, He, Xiao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

