Efficacy and Safety of Metformin Use in Rheumatoid Arthritis: A Randomized Controlled Study
- PMID: 34630103
- PMCID: PMC8493211
- DOI: 10.3389/fphar.2021.726490
Efficacy and Safety of Metformin Use in Rheumatoid Arthritis: A Randomized Controlled Study
Abstract
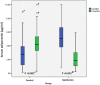
Objective: To evaluate the efficacy and safety of metformin use in rheumatoid arthritis (RA) patients receiving conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs). Methods: A prospective, randomized, controlled, single blinded, study was carried on 66 RA patients with moderate and high disease activity state, receiving csDMARDs. Patients were simply randomized to receive either metformin 850 mg twice daily (Metformin group, n = 33), or placebo twice daily (Control group, n = 33) in addition to their stable anti-rheumatic regimen and followed up for 6 months. Serum C-reactive protein (CRP), disease activity of 28 joints based on CRP (DAS-28-CRP), and quality of life (QOL) were evaluated at baseline and then every 3 months. Moreover, serum adiponectin was assessed at baseline and after 6 months. Results: Sixty patients completed the study. Drop out was due to intolerance to metformin side effects (n = 3) and non-compliance (n = 3). Metformin significantly decreased CRP levels and DAS-28-CRP after 6 months compared to the control group (p-value <0.001). A significant improvement in QOL of metformin group was observed as early as after 3 months (p-value = 0.006) with a continued improvement observed at 6 months (p-value <0.001) compared to the control group. Despite the significantly higher serum adiponectin in the metformin group at baseline, it was significantly reduced after 6 months in the metformin group with median percent change of -63.49% compared to the significant increase in the control group with median percent change of 92.40%. Conclusion: Metformin significantly improved inflammation, disease severity, and QOL in RA patients with high safety profile. Clinical Trial Registration: Clinical-Trials.gov, identifier [NCT08363405].
Keywords: CRP; DAS–28; adiponectin; metformin; quality of life; rheumatoid arthritis.
Copyright © 2021 Gharib, Elbaz, Darweesh, Sabri and Shawki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aletaha D., Neogi T., Silman A. J., Funovits J., Felson D. T., Bingham C. O., et al. (2010). 2010 Rheumatoid Arthritis Classification Criteria: an American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum. 62, 2569–2581. 10.1002/art.27584 - DOI - PubMed
-
- Castrejón I., Ortiz A. M., Toledano E., Castañeda S., García-vadillo A., Patiño E., et al. (2010). Estimated Cutoff Points for the 28-joint Disease Activity Score Based on C-Reactive Protein in a Longitudinal Register of Early Arthritis. J. Rheumatol. 37, 1439–1443. 10.3899/jrheum.091333 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

