Experimental Study of Almonertinib Crossing the Blood-Brain Barrier in EGFR-Mutant NSCLC Brain Metastasis and Spinal Cord Metastasis Models
- PMID: 34630120
- PMCID: PMC8497791
- DOI: 10.3389/fphar.2021.750031
Experimental Study of Almonertinib Crossing the Blood-Brain Barrier in EGFR-Mutant NSCLC Brain Metastasis and Spinal Cord Metastasis Models
Abstract
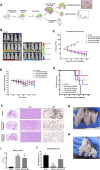
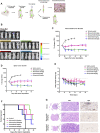
Roughly one third of non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)-sensitive mutated (EGFRm) tumors experience disease progression through central nervous system (CNS) metastases during treatment. Although EGFR-TKIs have been reported to be favored in some patients with EGFRm NSCLC CNS metastases, novel EGFR-TKIs with proven efficacy in CNS pathologies are clinically needed.To investigate whether almonertinib, a novel third-generation EGFR-TKI for NSCLC, can cross the blood-brain barrier (BBB) and deliver treatment for EGFR-mutant NSCLC brain metastases and spinal cord metastases, we constructed NSCLC brain metastasis and spinal cord metastasis models in vivo to observe the anti-tumor effects of almonertinib. Using ABCB1-MDCK and BCRP-MDCK monolayer cells as the in vitro study model, the effects of transport time and drug concentration on the apparent permeability coefficient of almonertinib and its active metabolite, HAS-719, were investigated. The results of this study show that almonertinib can significantly inhibit PC9 brain and spinal cord metastases. Pharmacokinetic studies in mice revealed that almonertinib has good BBB penetration ability, whereas the metabolite HAS-719 does not easily penetrate the BBB. Early clinical evidence of almonertinib activity in patients with EGFRm-advanced NSCLC and brain metastases has also been reported. In conclusion, almonertinib easily penetrates the BBB and inhibits advanced NSCLC brain and spinal cord metastases.
Keywords: EGFR-tyrosine kinase inhibitor; almonertinib; blood-brain barrier; brain and spinal cord metastases; non-small cell lung cancer; transmembrane resistance.
Copyright © 2021 Zhang, Zhang, Niu, Ge, Huang, Pang, Li, Wang, Gao, Fan, Li and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Restricting Glutamine Uptake Enhances NSCLC Sensitivity to Third-Generation EGFR-TKI Almonertinib.Front Pharmacol. 2021 May 14;12:671328. doi: 10.3389/fphar.2021.671328. eCollection 2021. Front Pharmacol. 2021. PMID: 34054543 Free PMC article.
-
Successful treatment of EGFR T790M-mutant non-small cell lung cancer with almonertinib after osimertinib-induced interstitial lung disease: a case report and literature review.Ann Transl Med. 2021 Jun;9(11):950. doi: 10.21037/atm-21-2823. Ann Transl Med. 2021. PMID: 34350265 Free PMC article.
-
Case report: Almonertinib in combination with bevacizumab for leptomeningeal metastases from epidermal growth factor receptor-mutation non-small cell lung cancer: Case series.Front Oncol. 2022 Nov 10;12:1040450. doi: 10.3389/fonc.2022.1040450. eCollection 2022. Front Oncol. 2022. PMID: 36439478 Free PMC article.
-
Management of CNS metastases in patients with EGFR mutation-positive NSCLC.Indian J Cancer. 2019 Nov;56(Supplement):S31-S37. doi: 10.4103/ijc.IJC_455_19. Indian J Cancer. 2019. PMID: 31793440 Review.
-
Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC).Lung Cancer. 2019 Nov;137:113-122. doi: 10.1016/j.lungcan.2019.09.017. Epub 2019 Sep 23. Lung Cancer. 2019. PMID: 31568888 Free PMC article. Review.
Cited by
-
Animal models of brain and spinal cord metastases of NSCLC established using a brain stereotactic instrument.Heliyon. 2024 Jan 20;10(3):e24809. doi: 10.1016/j.heliyon.2024.e24809. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38318004 Free PMC article.
-
Aumolertinib Effectively Reduces Clinical Symptoms of an EGFR L858R-Mutant Non-Small Cell Lung Cancer Case Coupled With Osimertinib-Induced Cardiotoxicity: Case Report and Review.Front Endocrinol (Lausanne). 2022 May 23;13:833929. doi: 10.3389/fendo.2022.833929. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35677717 Free PMC article. Review.
-
In vivo evaluation of the pharmacokinetic interactions between almonertinib and rivaroxaban, almonertinib and apixaban.Front Pharmacol. 2023 Oct 4;14:1263975. doi: 10.3389/fphar.2023.1263975. eCollection 2023. Front Pharmacol. 2023. PMID: 37860116 Free PMC article.
-
Aumolertinib: effective treatment for asymptomatic pulmonary giant cell carcinoma with EGFR L858R mutation - a case report.Front Oncol. 2023 Nov 27;13:1279045. doi: 10.3389/fonc.2023.1279045. eCollection 2023. Front Oncol. 2023. PMID: 38090500 Free PMC article.
-
Efficacy and safety of aumolertinib in EGFR-mutated non-small cell lung cancer with leptomeningeal metastasis: a single‑center retrospective study.J Neurooncol. 2025 Apr;172(2):461-470. doi: 10.1007/s11060-025-04938-w. Epub 2025 Feb 4. J Neurooncol. 2025. PMID: 39904875 Free PMC article.
References
-
- Ballard P., Yates J. W., Yang Z., Kim D. W., Yang J. C., Cantarini M., et al. (2016). Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin. Cancer Res. 22, 5130–5140. 10.1158/1078-0432.CCR-16-0399 - DOI - PubMed
-
- Borgelt B., Gelber R., Larson M., Hendrickson F., Griffin T., Roth R. (1981). Ultra-Rapid High Dose Irradiation Schedules for the Palliation of Brain Metastases: Final Results of the First Two Studies by the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 7, 1633–1638. 10.1016/0360-3016(81)90184-x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

