A Phase Defect Framework for the Analysis of Cardiac Arrhythmia Patterns
- PMID: 34630135
- PMCID: PMC8494009
- DOI: 10.3389/fphys.2021.690453
A Phase Defect Framework for the Analysis of Cardiac Arrhythmia Patterns
Abstract
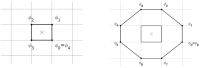
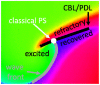
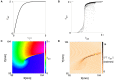
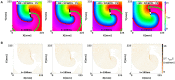
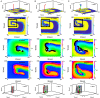
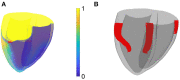
During cardiac arrhythmias, dynamical patterns of electrical activation form and evolve, which are of interest to understand and cure heart rhythm disorders. The analysis of these patterns is commonly performed by calculating the local activation phase and searching for phase singularities (PSs), i.e., points around which all phases are present. Here we propose an alternative framework, which focuses on phase defect lines (PDLs) and surfaces (PDSs) as more general mechanisms, which include PSs as a specific case. The proposed framework enables two conceptual unifications: between the local activation time and phase description, and between conduction block lines and the central regions of linear-core rotors. A simple PDL detection method is proposed and applied to data from simulations and optical mapping experiments. Our analysis of ventricular tachycardia in rabbit hearts (n = 6) shows that nearly all detected PSs were found on PDLs, but the PDLs had a significantly longer lifespan than the detected PSs. Since the proposed framework revisits basic building blocks of cardiac activation patterns, it can become a useful tool for further theory development and experimental analysis.
Keywords: cardiac arrhythmia; non-linear analysis; phase defect; self-organization; spiral wave.
Copyright © 2021 Arno, Quan, Nguyen, Vanmarcke, Tolkacheva and Dierckx.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





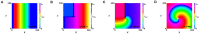


Similar articles
-
Rotors Detected by Phase Analysis of Filtered, Epicardial Atrial Fibrillation Electrograms Colocalize With Regions of Conduction Block.Circ Arrhythm Electrophysiol. 2018 Oct;11(10):e005858. doi: 10.1161/CIRCEP.117.005858. Circ Arrhythm Electrophysiol. 2018. PMID: 30354409 Free PMC article.
-
Detection Algorithm of Phase Singularity Using Phase Variance Analysis for Epicardial Optical Mapping Data.IEEE Trans Biomed Eng. 2016 Sep;63(9):1795-1803. doi: 10.1109/TBME.2015.2502726. Epub 2015 Nov 20. IEEE Trans Biomed Eng. 2016. PMID: 26599526
-
Interaction of phase singularities on the spiral wave tail: reconsideration of capturing the excitable gap.Am J Physiol Heart Circ Physiol. 2018 Aug 1;315(2):H318-H326. doi: 10.1152/ajpheart.00558.2017. Epub 2018 Mar 9. Am J Physiol Heart Circ Physiol. 2018. PMID: 29522372
-
Spiral wave activity: a possible common mechanism for polymorphic and monomorphic ventricular tachycardias.J Cardiovasc Electrophysiol. 1993 Dec;4(6):730-46. doi: 10.1111/j.1540-8167.1993.tb01258.x. J Cardiovasc Electrophysiol. 1993. PMID: 8305992 Review.
-
Demystifying rotors and their place in clinical translation of atrial fibrillation mechanisms.Nat Rev Cardiol. 2017 Sep;14(9):509-520. doi: 10.1038/nrcardio.2017.37. Epub 2017 Apr 6. Nat Rev Cardiol. 2017. PMID: 28383023 Review.
Cited by
-
A comprehensive framework for evaluation of high pacing frequency and arrhythmic optical mapping signals.Front Physiol. 2023 Jan 23;14:734356. doi: 10.3389/fphys.2023.734356. eCollection 2023. Front Physiol. 2023. PMID: 36755791 Free PMC article.
-
Numerical methods for the detection of phase defect structures in excitable media.PLoS One. 2022 Jul 12;17(7):e0271351. doi: 10.1371/journal.pone.0271351. eCollection 2022. PLoS One. 2022. PMID: 35819963 Free PMC article.
-
Impact of electrode orientation, myocardial wall thickness, and myofiber direction on intracardiac electrograms: numerical modeling and analytical solutions.Front Physiol. 2023 Jul 10;14:1213218. doi: 10.3389/fphys.2023.1213218. eCollection 2023. Front Physiol. 2023. PMID: 37492643 Free PMC article.
-
The Ithildin library for efficient numerical solution of anisotropic reaction-diffusion problems in excitable media.PLoS One. 2024 Sep 19;19(9):e0303674. doi: 10.1371/journal.pone.0303674. eCollection 2024. PLoS One. 2024. PMID: 39298417 Free PMC article.
-
Analysis of complex excitation patterns using Feynman-like diagrams.Sci Rep. 2024 Nov 22;14(1):28962. doi: 10.1038/s41598-024-73544-z. Sci Rep. 2024. PMID: 39578507 Free PMC article.
References
-
- Aliev R., Panfilov A. (1996). A simple two-variable model of cardiac excitation. Chaos Solitons Fractals 7, 293–301. 10.1016/0960-0779(95)00089-5 - DOI
-
- Arfken G. B., Weber H. J. (1995). Mathematical Methods for Physicists, 4th Edn. San Diego, CA: Academic Press.
-
- Arno L., Quan J., Vanmarcke M., Nguyen N., Tolkacheva E., Dierckx H. (2021). Phase defect lines during cardiac arrhythmias: from theory to experiment. arXiv [Preprint] arXiv:2101.00315. Available online at: https://arxiv.org/abs/2101.00315v1
LinkOut - more resources
Full Text Sources

