Long-Lasting Desynchronization Effects of Coordinated Reset Stimulation Improved by Random Jitters
- PMID: 34630142
- PMCID: PMC8497886
- DOI: 10.3389/fphys.2021.719680
Long-Lasting Desynchronization Effects of Coordinated Reset Stimulation Improved by Random Jitters
Abstract
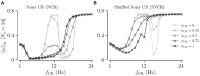
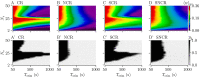
Abnormally strong synchronized activity is related to several neurological disorders, including essential tremor, epilepsy, and Parkinson's disease. Chronic high-frequency deep brain stimulation (HF DBS) is an established treatment for advanced Parkinson's disease. To reduce the delivered integral electrical current, novel theory-based stimulation techniques such as coordinated reset (CR) stimulation directly counteract the abnormal synchronous firing by delivering phase-shifted stimuli through multiple stimulation sites. In computational studies in neuronal networks with spike-timing-dependent plasticity (STDP), it was shown that CR stimulation down-regulates synaptic weights and drives the network into an attractor of a stable desynchronized state. This led to desynchronization effects that outlasted the stimulation. Corresponding long-lasting therapeutic effects were observed in preclinical and clinical studies. Computational studies suggest that long-lasting effects of CR stimulation depend on the adjustment of the stimulation frequency to the dominant synchronous rhythm. This may limit clinical applicability as different pathological rhythms may coexist. To increase the robustness of the long-lasting effects, we study randomized versions of CR stimulation in networks of leaky integrate-and-fire neurons with STDP. Randomization is obtained by adding random jitters to the stimulation times and by shuffling the sequence of stimulation site activations. We study the corresponding long-lasting effects using analytical calculations and computer simulations. We show that random jitters increase the robustness of long-lasting effects with respect to changes of the number of stimulation sites and the stimulation frequency. In contrast, shuffling does not increase parameter robustness of long-lasting effects. Studying the relation between acute, acute after-, and long-lasting effects of stimulation, we find that both acute after- and long-lasting effects are strongly determined by the stimulation-induced synaptic reshaping, whereas acute effects solely depend on the statistics of administered stimuli. We find that the stimulation duration is another important parameter, as effective stimulation only entails long-lasting effects after a sufficient stimulation duration. Our results show that long-lasting therapeutic effects of CR stimulation with random jitters are more robust than those of regular CR stimulation. This might reduce the parameter adjustment time in future clinical trials and make CR with random jitters more suitable for treating brain disorders with abnormal synchronization in multiple frequency bands.
Keywords: coordinated reset stimulation; long-lasting desynchronization; random jitter; spike-timing-dependent plasticity (STDP); stimulation-induced decoupling.
Copyright © 2021 Khaledi-Nasab, Kromer and Tass.
Conflict of interest statement
PT works as a consultant for Boston Scientific Neuromodulation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Coordinated reset stimulation of plastic neural networks with spatially dependent synaptic connections.Front Netw Physiol. 2024 May 28;4:1351815. doi: 10.3389/fnetp.2024.1351815. eCollection 2024. Front Netw Physiol. 2024. PMID: 38863734 Free PMC article.
-
Long-Lasting Desynchronization of Plastic Neuronal Networks by Double-Random Coordinated Reset Stimulation.Front Netw Physiol. 2022 Apr 19;2:864859. doi: 10.3389/fnetp.2022.864859. eCollection 2022. Front Netw Physiol. 2022. PMID: 36926109 Free PMC article.
-
Impact of number of stimulation sites on long-lasting desynchronization effects of coordinated reset stimulation.Chaos. 2020 Aug;30(8):083134. doi: 10.1063/5.0015196. Chaos. 2020. PMID: 32872805
-
Delayed and lasting effects of deep brain stimulation on locomotion in Parkinson's disease.Chaos. 2009 Jun;19(2):026114. doi: 10.1063/1.3127585. Chaos. 2009. PMID: 19566274 Review.
-
Maladaptive neural synchrony in tinnitus: origin and restoration.Front Neurol. 2015 Feb 17;6:29. doi: 10.3389/fneur.2015.00029. eCollection 2015. Front Neurol. 2015. PMID: 25741316 Free PMC article. Review.
Cited by
-
Dynamics of phase oscillator networks with synaptic weight and structural plasticity.Sci Rep. 2022 Sep 2;12(1):15003. doi: 10.1038/s41598-022-19417-9. Sci Rep. 2022. PMID: 36056151 Free PMC article.
-
Decoupling of interacting neuronal populations by time-shifted stimulation through spike-timing-dependent plasticity.PLoS Comput Biol. 2023 Feb 1;19(2):e1010853. doi: 10.1371/journal.pcbi.1010853. eCollection 2023 Feb. PLoS Comput Biol. 2023. PMID: 36724144 Free PMC article.
-
Synaptic reshaping of plastic neuronal networks by periodic multichannel stimulation with single-pulse and burst stimuli.PLoS Comput Biol. 2022 Nov 3;18(11):e1010568. doi: 10.1371/journal.pcbi.1010568. eCollection 2022 Nov. PLoS Comput Biol. 2022. PMID: 36327232 Free PMC article.
-
Coordinated reset stimulation of plastic neural networks with spatially dependent synaptic connections.Front Netw Physiol. 2024 May 28;4:1351815. doi: 10.3389/fnetp.2024.1351815. eCollection 2024. Front Netw Physiol. 2024. PMID: 38863734 Free PMC article.
-
Synaptic reorganization of synchronized neuronal networks with synaptic weight and structural plasticity.PLoS Comput Biol. 2024 Jul 9;20(7):e1012261. doi: 10.1371/journal.pcbi.1012261. eCollection 2024 Jul. PLoS Comput Biol. 2024. PMID: 38980898 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

