Rheological Impact of GBT1118 Cessation in a Sickle Mouse Model
- PMID: 34630162
- PMCID: PMC8497897
- DOI: 10.3389/fphys.2021.742784
Rheological Impact of GBT1118 Cessation in a Sickle Mouse Model
Abstract
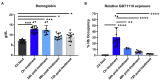
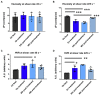
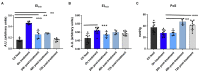
In sickle cell disease (SCD), higher whole blood viscosity is a risk factor for vaso-occlusive crisis, avascular necrosis, and proliferative retinopathy. Blood viscosity is strongly impacted by hemoglobin (Hb) levels and red blood cell (RBC) deformability. Voxelotor is a hemoglobin S (HbS) polymerization inhibitor with anti-sickling properties that increases the Hb affinity for oxygen, thereby reducing HbS polymerization. In clinical trials, voxelotor increased Hb by an average of 1g/dl, creating concern that this rise in Hb could increase viscosity, particularly when the drug was cleared. To investigate this potential rebound hyperviscosity effect, we treated SCD mice with GBT1118, a voxelotor analog, and stopped the treatment to determine the effect on blood viscosity and RBC deformability under a range of oxygen concentrations. GBT1118 treatment increased Hb, improved RBC deformability by increasing the elongation index under normoxic (EImax) and hypoxic conditions (EImin), and decreased the point of sickling (PoS) without increasing blood viscosity. The anti-sickling effects and improvement of RBC deformability balanced the effect of increased Hb such that there was no increase in blood viscosity. Forty-eight hours after ceasing GBT1118, Hb declined from the rise induced by treatment, viscosity did not increase, and EImin remained elevated compared to control animals. Hb and PoS were not different from control animals, suggesting a return to native oxygen affinity and clearance of the drug. RBC deformability did not return to baseline, suggesting some residual rheological improvement. These data suggest that concerns regarding viscosity rise above pre-treatment levels upon sudden cessation of voxelotor are not warranted.
Keywords: GBT1118; deformability; hemorheology; red cell; rheological biomarkers; sickle cell disease; viscosity; voxelotor.
Copyright © 2021 Kanne, Nebor, Pochron, Oksenberg and Sheehan.
Conflict of interest statement
MP – Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. DO – Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. VS – Global Blood Therapeutics: Research Funding. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
GBT440 improves red blood cell deformability and reduces viscosity of sickle cell blood under deoxygenated conditions.Clin Hemorheol Microcirc. 2018;70(1):95-105. doi: 10.3233/CH-170340. Clin Hemorheol Microcirc. 2018. PMID: 29660913 Free PMC article.
-
Increased hemoglobin affinity for oxygen with GBT1118 improves hypoxia tolerance in sickle cell mice.Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H400-H411. doi: 10.1152/ajpheart.00048.2021. Epub 2021 Jul 2. Am J Physiol Heart Circ Physiol. 2021. PMID: 34213392 Free PMC article.
-
GBT1118, a voxelotor analog, protects red blood cells from damage during severe hypoxia.Am J Transl Res. 2022 Jan 15;14(1):240-251. eCollection 2022. Am J Transl Res. 2022. PMID: 35173841 Free PMC article.
-
Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise.Front Physiol. 2019 Oct 17;10:1329. doi: 10.3389/fphys.2019.01329. eCollection 2019. Front Physiol. 2019. PMID: 31749708 Free PMC article. Review.
-
Osivelotor for the treatment of sickle cell disease.Expert Opin Pharmacother. 2025 May;26(7):801-808. doi: 10.1080/14656566.2025.2489123. Epub 2025 Apr 6. Expert Opin Pharmacother. 2025. PMID: 40179004 Review.
Cited by
-
GBT1118, a Voxelotor Analog, Ameliorates Hepatopathy in Sickle Cell Disease.Medicina (Kaunas). 2024 Sep 26;60(10):1581. doi: 10.3390/medicina60101581. Medicina (Kaunas). 2024. PMID: 39459368 Free PMC article.
-
Time to rethink haemoglobin threshold guidelines in sickle cell disease.Br J Haematol. 2021 Nov;195(4):518-522. doi: 10.1111/bjh.17578. Epub 2021 Jun 15. Br J Haematol. 2021. PMID: 34131897 Free PMC article.
-
Mouse models of sickle cell disease: Imperfect and yet very informative.Blood Cells Mol Dis. 2024 Jan;104:102776. doi: 10.1016/j.bcmd.2023.102776. Epub 2023 Jun 17. Blood Cells Mol Dis. 2024. PMID: 37391346 Free PMC article. Review.
References
-
- Adams R. J., Mckie V. C., Hsu L., Files B., Vichinsky E., Pegelow C., et al. (1998). Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N. Engl. J. Med. 339, 5–11. doi: 10.1056/NEJM199807023390102 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

