Structural and Mechanistic Insights Into Dimethylsulfoxide Formation Through Dimethylsulfide Oxidation
- PMID: 34630359
- PMCID: PMC8498191
- DOI: 10.3389/fmicb.2021.735793
Structural and Mechanistic Insights Into Dimethylsulfoxide Formation Through Dimethylsulfide Oxidation
Abstract
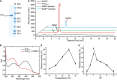
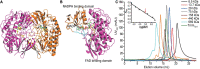
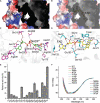
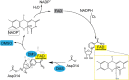
Dimethylsulfide (DMS) and dimethylsulfoxide (DMSO) are widespread in marine environment, and are important participants in the global sulfur cycle. Microbiol oxidation of DMS to DMSO represents a major sink of DMS in marine surface waters. The SAR11 clade and the marine Roseobacter clade (MRC) are the most abundant heterotrophic bacteria in the ocean surface seawater. It has been reported that trimethylamine monooxygenase (Tmm, EC 1.14.13.148) from both MRC and SAR11 bacteria likely oxidizes DMS to generate DMSO. However, the structural basis of DMS oxidation has not been explained. Here, we characterized a Tmm homolog from the SAR11 bacterium Pelagibacter sp. HTCC7211 (Tmm7211). Tmm7211 exhibits DMS oxidation activity in vitro. We further solved the crystal structures of Tmm7211 and Tmm7211 soaked with DMS, and proposed the catalytic mechanism of Tmm7211, which comprises a reductive half-reaction and an oxidative half-reaction. FAD and NADPH molecules are essential for the catalysis of Tmm7211. In the reductive half-reaction, FAD is reduced by NADPH. In the oxidative half-reaction, the reduced FAD reacts with O2 to form the C4a-(hydro)peroxyflavin. The binding of DMS may repel the nicotinamide ring of NADP+, and make NADP+ generate a conformational change, shutting off the substrate entrance and exposing the active C4a-(hydro)peroxyflavin to DMS to complete the oxidation of DMS. The proposed catalytic mechanism of Tmm7211 may be widely adopted by MRC and SAR11 bacteria. This study provides important insight into the conversion of DMS into DMSO in marine bacteria, leading to a better understanding of the global sulfur cycle.
Keywords: DMS; DMSO; SAR11; catalytic mechanism; flavin-containing monooxygenase.
Copyright © 2021 Wang, Zhang, Teng, Wang, Zhang, Chen, Zhang, Chen, Fu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Andreae M. O. (1990). Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar. Chem. 30 1–29. 10.1016/0304-4203(90)90059-l - DOI
-
- Asher E. C., Dacey J. W. H., Stukel M., Long M. C., Tortell P. D. (2017). Processes driving seasonal variability in DMS, DMSP, and DMSO concentrations and turnover in coastal Antarctic waters. Limnol. Oceanogr. 62 104–124. 10.1002/lno.10379 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

