The bZIP Transcription Factor LtAP1 Modulates Oxidative Stress Tolerance and Virulence in the Peach Gummosis Fungus Lasiodiplodia theobromae
- PMID: 34630367
- PMCID: PMC8495313
- DOI: 10.3389/fmicb.2021.741842
The bZIP Transcription Factor LtAP1 Modulates Oxidative Stress Tolerance and Virulence in the Peach Gummosis Fungus Lasiodiplodia theobromae
Abstract
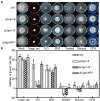
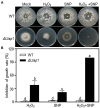
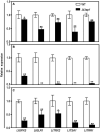
Lasiodiplodia theobromae is one of the primary causal agents in peach gummosis disease, leading to enormous losses in peach production. In our previous study, a redox-related gene, LtAP1, from the fungus was significantly upregulated in peach shoots throughout infection. Here, we characterized LtAP1, a basic leucine zipper transcription factor, during peach gummosis progression using the CRISPR-Cas9 system and homologous recombination. The results showed that LtAP1-deletion mutant had slower vegetative growth and increased sensitivity to several oxidative and nitrosative stress agents. LtAP1 was highly induced by exogenous oxidants treatment in the L. theobromae wild-type strain. In a pathogenicity test, the deletion mutant showed decreased virulence (reduced size of necrotic lesions, less gum release, and decreased pathogen biomass) on infected peach shoots compared to the wild-type strain. The mutant showed severely reduced transcription levels of genes related to glutaredoxin and thioredoxin in L. theobroame under oxidative stress or during infection, indicating an attenuated capacity for reactive oxygen species (ROS) detoxification. When shoots were treated with an NADPH oxidase inhibitor, the pathogenicity of the mutant was partially restored. Moreover, ROS production and plant defense response were strongly activated in peach shoots infected by the mutant. These results highlight the crucial role of LtAP1 in the oxidative stress response, and further that it acts as an important virulence factor through modulating the fungal ROS-detoxification system and the plant defense response.
Keywords: AP1 transcription factor; Lasiodiplodia theobromae; fungal virulence; oxidative stress response; peach gummosis disease; plant defense response.
Copyright © 2021 Zhang, Shen, Zhang, Shen, Wang, Hsiang, Liu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Alves A., Crous P. W., Correia A., Phillips A. J. L. (2008). Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 28, 1–13. doi: 10.1002/yea.1554 - DOI
-
- Beckman T. G., Pusey P. L., Bertrand P. F. (2003). Impact of fungal gummosis on peach trees. HortScience 38, 1141–1143. doi: 10.21273/HORTSCI.38.6.1141 - DOI
LinkOut - more resources
Full Text Sources

