Comprehensive Immune Profiling of a Kidney Transplant Recipient With Peri-Operative SARS-CoV-2 Infection: A Case Report
- PMID: 34630432
- PMCID: PMC8492986
- DOI: 10.3389/fimmu.2021.753558
Comprehensive Immune Profiling of a Kidney Transplant Recipient With Peri-Operative SARS-CoV-2 Infection: A Case Report
Abstract
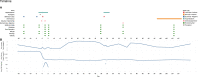
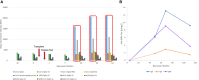
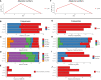
To date there is limited data on the immune profile and outcomes of solid organ transplant recipients who encounter COVID-19 infection early post-transplant. Here we present a unique case where the kidney recipient's transplant surgery coincided with a positive SARS-CoV-2 test and the patient subsequently developed symptomatic COVID-19 perioperatively. We performed comprehensive immunological monitoring of cellular, proteomic, and serological changes during the first 4 critical months post-infection. We showed that continuation of basiliximab induction and maintenance of triple immunosuppression did not significantly impair the host's ability to mount a robust immune response against symptomatic COVID-19 infection diagnosed within the first week post-transplant.
Keywords: COVID-19; immune response; immunosuppressants; induction therapy; transplant.
Copyright © 2021 Sherwood, Nicholl, Fenninger, Wu, Wong, Benedicto, Cina, Wang, Pobran, De Marco, Márquez, Jassem, Sekirov, Morshed, Bardi, Sekhon, Keown, Kadatz and Lan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

