Protective Effect of an Anti-HMGB-1 Neutralizing Antibody on Hemozoin-Induced Alveolar Epithelial Cell in a Model of Malaria Associated ALI/ARDS
- PMID: 34630581
- PMCID: PMC8476737
- DOI: 10.18502/ijpa.v16i3.7089
Protective Effect of an Anti-HMGB-1 Neutralizing Antibody on Hemozoin-Induced Alveolar Epithelial Cell in a Model of Malaria Associated ALI/ARDS
Abstract
Background: We aimed to determine whether neutralizing high mobility group box-1 (HMGB-1) prevents the release of HMGB-1 and proinflammatory cytokines on hemozoin (Hz)-induced alveolar epithelial cell in a model of malaria associated ALI/ARDS.
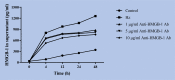
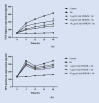
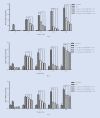
Methods: This study was conducted in the Department of Tropical Pathology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand in 2020. Human pulmonary alveolar epithelial cells (HPAEpiCs) were exposed to medium alone or 20 μM Hz for 24 h and incubated with different concentrations (1, 5, and 10 μg/ml) of anti-HMGB-1 monoclonal antibody (mAb) for various times (0, 4, 12, 24, and 48 h). The levels of HMGB-1, TNF-α and IFN-γ in the supernatants were measured by ELISA. The mRNA expression of RAGE, TLR-2 and TLR-4 were analyzed by real-time PCR.
Results: The HPAEpiCs treated with 10 μg/ml anti-HMGB-1 mAb showed a significant reduction in HMGB-1 release into the supernatant compared with those treated with 1 and 5 μg/ml anti-HMGB-1 mAb. The levels of TNF-α and IFN-γ were significantly decreased in the supernatant of HPAEpiCs treated with 1, 5, and 10 μg/ml anti-HMGB-1 mAb for 4, 12, 24, and 48 h compared with those stimulated with Hz alone. The mRNA expression levels of RAGE, TLR-2, and TLR-4 were significantly decreased after 24 h of anti-HMGB-1 antibody treatment at all concentrations.
Conclusion: An anti-HMGB-1 antibody could be an effective agent for inhibiting the release of HMGB-1, TNF-α and IFN-γ. Furthermore, a neutralizing anti-HMGB-1 antibody could be applicable for the treatment of malaria-associated ALI/ARDS.
Keywords: Acute lung injury; HMGB1 protein; Malaria.
Copyright © 2021 Techarang et al. Published by Tehran University of Medical Sciences.
Conflict of interest statement
Conflict of interest The authors declared that there is no conflict of interest.
Figures
References
-
- Mohan A, Sharma S, Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. J Vector Borne Dis. 2008; 45(3):179–93. - PubMed
-
- Deroost K, Tyberghein A, Lays N, et al. Hemozoin induces lung inflammation and correlates with malaria-associated acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2013; 48(5):589–600. - PubMed
LinkOut - more resources
Full Text Sources
