Interleukin-17 activates JAK2/STAT3, PI3K/Akt and nuclear factor-κB signaling pathway to promote the tumorigenesis of cervical cancer
- PMID: 34630646
- PMCID: PMC8461522
- DOI: 10.3892/etm.2021.10726
Interleukin-17 activates JAK2/STAT3, PI3K/Akt and nuclear factor-κB signaling pathway to promote the tumorigenesis of cervical cancer
Abstract
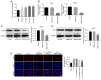
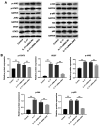
Interleukin (IL)-17 has been regarded as a significant factor in inflammation. In addition, IL-17 is known to be involved in the progression of cancers; however, the function of IL-17 in cervical cancer remains unclear. In the present study, cell viability was detected by Cell Counting Kit-8 assay. Quantitative PCR and western blotting were performed to detect gene and protein expression levels, respectively, in cancer cells or tissues. Ki-67 staining was used to evaluate cell proliferation. Wound-healing assay was used to detect cell migration. Moreover, Transwell assay was performed to investigate the invasion of cervical cancer cells. The results revealed that IL-17 significantly promoted the proliferation of cervical cancer cells. Additionally, IL-17 notably enhanced the migration and invasion of cervical cancer cells in vitro. IL-17 promoted the progression of cervical cancer via the activation of JAK2/STAT3 and PI3K/Akt/NF-κB signaling. In conclusion, IL-17 was a key regulator during the progression of cervical cancer through the JAK2/STAT3 and PI3K/Akt/nuclear factor-κB signaling pathway, which may serve as a novel target for the treatment of cervical cancer.
Keywords: JAK2/STAT3; PI3K/Akt; cervical cancer; interleukin-17; nuclear factor-κB.
Copyright: © Bai et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, Bothou A, Galazios G. Cervical cancer: Screening, diagnosis and staging. J BUON. 2016;21:320–325. - PubMed
-
- Zeng SY, Liang MR, Li LY, Li L, Jiang W, Zhong ML. Application of transvaginal external fascia trachelectomy in the treatment of CIN and micro-invasive cervical cancer. Zhonghua Zhong Liu Za Zhi. 2013;35:543–546. (In Chinese) - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
