Study of the Possible Alleviated Role of Atorvastatin on Irinotecan-Induced Lingual Mucosal Damage: Histological and Molecular Study
- PMID: 34630855
- PMCID: PMC8497104
- DOI: 10.1155/2021/9690047
Study of the Possible Alleviated Role of Atorvastatin on Irinotecan-Induced Lingual Mucosal Damage: Histological and Molecular Study
Abstract
Background: Oral mucositis is the most debilitating and troublesome adverse effect of irinotecan (CPT-11) treatment. It adversely affects the patient quality of life. The aim of this work was to study the histological, immunohistochemical, and molecular changes in the oral mucosa by CPT-11 and the possible alleviated role of atorvastatin.
Methods: Rats were randomly divided into control, CPT-11-treated group, and CPT-11+ atorvastatin-treated group. At the end of the experiment, the anterior two-thirds of the tongue was dissected out and divided into two parts: one part for light microscopic examination and the second for molecular study.
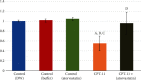
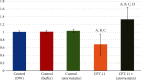
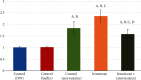
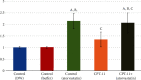
Results: CPT-11-treated group revealed loss of normal mucosal organization, areas of ulceration and inflammation, and loss of architecture of lingual papillae. A significant decrease in immunohistochemical and molecular gene expression of Ki-67 and antiapoptotic Bcl-2 levels was observed. A significant increase in NF-κB immunohistochemical and mRNA gene expression level and a nonsignificant increase in Nrf2 gene expression were detected. Coadministration of atorvastatin showed remarkable improvement in the histopathological picture with a significant increase in Ki-67 and Bcl-2, a significant decrease in NF-κB protein and gene expression, and a significant increase in Nrf2 gene expression.
Conclusion: Atorvastatin substantially attenuates CPT-11-induced oral mucositis through the initiation of the antiapoptotic gene, modulation of the inflammatory, and antioxidant gene expression.
Copyright © 2021 Eetmad A. Arafat et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
-
- Anderson M. K., Matey L. Irinotecan: 25 years of cancer treatment. Overview of cancer and cancer treatment. In: Olsen M. M., KB L. F., Brassil K. J., editors. Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice . Pittsburgh, PA.: Oncology Nursing Society; 2019.
-
- Miyano K., Eto M., Hitomi S., et al. Author correction: the Japanese herbal medicine Hangeshashinto enhances oral keratinocyte migration to facilitate healing of chemotherapy-induced oral ulcerative mucositis. Scientific Reports . 2020;10(1, article 19806) doi: 10.1038/s41598-020-76581-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

