Mathematical model of STAT signalling pathways in cancer development and optimal control approaches
- PMID: 34631119
- PMCID: PMC8479343
- DOI: 10.1098/rsos.210594
Mathematical model of STAT signalling pathways in cancer development and optimal control approaches
Abstract
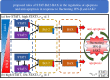
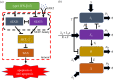
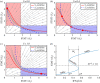
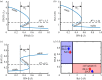
In various diseases, the STAT family display various cellular controls over various challenges faced by the immune system and cell death programs. In this study, we investigate how an intracellular signalling network (STAT1, STAT3, Bcl-2 and BAX) regulates important cellular states, either anti-apoptosis or apoptosis of cancer cells. We adapt a mathematical framework to illustrate how the signalling network can generate a bi-stability condition so that it will induce either apoptosis or anti-apoptosis status of tumour cells. Then, we use this model to develop several anti-tumour strategies including IFN-β infusion. The roles of JAK-STATs signalling in regulation of the cell death program in cancer cells and tumour growth are poorly understood. The mathematical model unveils the structure and functions of the intracellular signalling and cellular outcomes of the anti-tumour drugs in the presence of IFN-β and JAK stimuli. We identify the best injection order of IFN-β and DDP among many possible combinations, which may suggest better infusion strategies of multiple anti-cancer agents at clinics. We finally use an optimal control theory in order to maximize anti-tumour efficacy and minimize administrative costs. In particular, we minimize tumour volume and maximize the apoptotic potential by minimizing the Bcl-2 concentration and maximizing the BAX level while minimizing total injection amount of both IFN-β and JAK2 inhibitors (DDP).
Keywords: STAT1; apoptosis; cancer; mathematical model; optimal control.
© 2021 The Authors.
Figures


Similar articles
-
Optimal regulation of tumour-associated neutrophils in cancer progression.R Soc Open Sci. 2022 Feb 2;9(2):210705. doi: 10.1098/rsos.210705. eCollection 2022 Feb. R Soc Open Sci. 2022. PMID: 35127110 Free PMC article.
-
HIV-1 Promotes the Degradation of Components of the Type 1 IFN JAK/STAT Pathway and Blocks Anti-viral ISG Induction.EBioMedicine. 2018 Apr;30:203-216. doi: 10.1016/j.ebiom.2018.03.006. Epub 2018 Mar 9. EBioMedicine. 2018. PMID: 29580840 Free PMC article.
-
The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours.Br J Cancer. 2015 Jul 28;113(3):365-71. doi: 10.1038/bjc.2015.233. Epub 2015 Jul 7. Br J Cancer. 2015. PMID: 26151455 Free PMC article.
-
The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus.J Recept Signal Transduct Res. 1999 Jan-Jul;19(1-4):75-120. doi: 10.3109/10799899909036638. J Recept Signal Transduct Res. 1999. PMID: 10071751 Review.
-
STAT proteins as novel targets for cancer drug discovery.Expert Opin Ther Targets. 2004 Oct;8(5):409-22. doi: 10.1517/14728222.8.5.409. Expert Opin Ther Targets. 2004. PMID: 15469392 Review.
Cited by
-
Order-of-Mutation Effects on Cancer Progression: Models for Myeloproliferative Neoplasm.Bull Math Biol. 2024 Feb 16;86(3):32. doi: 10.1007/s11538-024-01257-5. Bull Math Biol. 2024. PMID: 38363386 Free PMC article.
-
Mathematical Modeling Support for Lung Cancer Therapy-A Short Review.Int J Mol Sci. 2023 Sep 25;24(19):14516. doi: 10.3390/ijms241914516. Int J Mol Sci. 2023. PMID: 37833963 Free PMC article. Review.
-
Optimal regulation of tumour-associated neutrophils in cancer progression.R Soc Open Sci. 2022 Feb 2;9(2):210705. doi: 10.1098/rsos.210705. eCollection 2022 Feb. R Soc Open Sci. 2022. PMID: 35127110 Free PMC article.
-
Predicting gene-level sensitivity to JAK-STAT signaling perturbation using a mechanistic-to-machine learning framework.Cell Syst. 2024 Jan 17;15(1):37-48.e4. doi: 10.1016/j.cels.2023.12.006. Epub 2024 Jan 9. Cell Syst. 2024. PMID: 38198893 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

