Organ-differential Roles of Akt/FoxOs Axis as a Key Metabolic Modulator during Aging
- PMID: 34631216
- PMCID: PMC8460295
- DOI: 10.14336/AD.2021.0225
Organ-differential Roles of Akt/FoxOs Axis as a Key Metabolic Modulator during Aging
Abstract
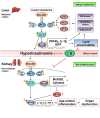
FoxOs and their post-translational modification by phosphorylation, acetylation, and methylation can affect epigenetic modifications and promote the expression of downstream target genes. Therefore, they ultimately affect cellular and biological functions during aging or occurrence of age-related diseases including cancer, diabetes, and kidney diseases. As known for its key role in aging, FoxOs play various biological roles in the aging process by regulating reactive oxygen species, lipid accumulation, and inflammation. FoxOs regulated by PI3K/Akt pathway modulate the expression of various target genes encoding MnSOD, catalases, PPARγ, and IL-1β during aging, which are associated with age-related diseases. This review highlights the age-dependent differential regulatory mechanism of Akt/FoxOs axis in metabolic and non-metabolic organs. We demonstrated that age-dependent suppression of Akt increases the activity of FoxOs (Akt/FoxOs axis upregulation) in metabolic organs such as liver and muscle. This Akt/FoxOs axis could be modulated and reversed by antiaging paradigm calorie restriction (CR). In contrast, hyperinsulinemia-mediated PI3K/Akt activation inhibited FoxOs activity (Akt/FoxOs axis downregulation) leading to decrease of antioxidant genes expression in non-metabolic organs such as kidneys and lungs during aging. These phenomena are reversed by CR. The results of studies on the process of aging and CR indicate that the Akt/FoxOs axis plays a critical role in regulating metabolic homeostasis, redox stress, and inflammation in various organs during aging process. The benefical actions of CR on the Akt/FoxOs axis in metabolic and non-metabolic organs provide further insights into the molecular mechanisms of organ-differential roles of Akt/FoxOs axis during aging.
Keywords: Aging; Akt/FoxOs axis; CR; inflammation; metabolic organs; non-metabolic organs.
copyright: © 2021 Kim et al.
Conflict of interest statement
Conflict of interest The authors have no conflicts of interests.
Figures
Similar articles
-
The roles of FoxOs in modulation of aging by calorie restriction.Biogerontology. 2015 Feb;16(1):1-14. doi: 10.1007/s10522-014-9519-y. Epub 2014 Aug 22. Biogerontology. 2015. PMID: 25146189 Review.
-
Akt, FoxO and regulation of apoptosis.Biochim Biophys Acta. 2011 Nov;1813(11):1978-86. doi: 10.1016/j.bbamcr.2011.03.010. Epub 2011 Mar 31. Biochim Biophys Acta. 2011. PMID: 21440011 Review.
-
The essential role of FoxO6 phosphorylation in aging and calorie restriction.Age (Dordr). 2014;36(4):9679. doi: 10.1007/s11357-014-9679-3. Epub 2014 Jul 10. Age (Dordr). 2014. PMID: 25007762 Free PMC article.
-
Insulin/IGF-1R, SIRT1, and FOXOs Pathways-An Intriguing Interaction Platform for Bone and Osteosarcoma.Front Endocrinol (Lausanne). 2019 Mar 1;10:93. doi: 10.3389/fendo.2019.00093. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30881341 Free PMC article.
-
Redox regulation of FoxO transcription factors.Redox Biol. 2015 Dec;6:51-72. doi: 10.1016/j.redox.2015.06.019. Epub 2015 Jul 3. Redox Biol. 2015. PMID: 26184557 Free PMC article. Review.
Cited by
-
Dynamics of serum exosome microRNA profile altered by chemically induced estropause and rescued by estrogen therapy in female mice.Geroscience. 2024 Dec;46(6):5891-5909. doi: 10.1007/s11357-024-01129-9. Epub 2024 Mar 19. Geroscience. 2024. PMID: 38499957 Free PMC article.
-
The effect of Astragali Radix-Radix Angelica Sinensis on acute kidney injury: a network pharmacology and molecular docking study.Transl Androl Urol. 2024 Jan 31;13(1):91-103. doi: 10.21037/tau-23-562. Epub 2024 Jan 23. Transl Androl Urol. 2024. PMID: 38404557 Free PMC article.
-
Understanding chronic inflammation: couplings between cytokines, ROS, NO, Cai 2+, HIF-1α, Nrf2 and autophagy.Front Immunol. 2025 Apr 8;16:1558263. doi: 10.3389/fimmu.2025.1558263. eCollection 2025. Front Immunol. 2025. PMID: 40264757 Free PMC article. Review.
-
Molecular constraints of sarcopenia in the ageing muscle.Front Aging. 2025 Jul 3;6:1588014. doi: 10.3389/fragi.2025.1588014. eCollection 2025. Front Aging. 2025. PMID: 40678078 Free PMC article. Review.
-
Restoring gluconeogenesis by TEF inhibited proliferation and promoted apoptosis and immune surveillance in kidney renal clear cell carcinoma.Cancer Metab. 2023 Aug 8;11(1):11. doi: 10.1186/s40170-023-00312-4. Cancer Metab. 2023. PMID: 37553601 Free PMC article.
References
-
- Krasilnikov MA (2000). Phosphatidylinositol-3 kinase dependent pathways: The role in control of cell growth, survival, and malignant transformation. Biochemistry, 65:59-67. - PubMed
-
- Jeong H, Liu Y, Kim HS (2017). Dried plum and chokeberry ameliorate d-galactose-induced aging in mice by regulation of Pl3k/Akt-mediated Nrf2 and Nf-kB pathways. Exp Gerontol, 95:16-25. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
