HIF-1α promotes the migration and invasion of cancer-associated fibroblasts by miR-210
- PMID: 34631221
- PMCID: PMC8460292
- DOI: 10.14336/AD.2021.0315
HIF-1α promotes the migration and invasion of cancer-associated fibroblasts by miR-210
Abstract
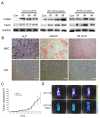
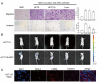
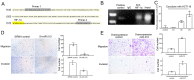
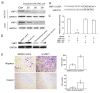
Metastasis is the major cause of death in colorectal cancer (CRC) patients. Inhibition of metastasis will prolong the survival of patients with CRC. Cancer cells bring their own soil, cancer-associated fibroblasts (CAFs), to metastasize together, promoting the survival and colonization of circulating cancer cells. However, the mechanism by which CAFs metastasize remains unclear. In this study, CAFs were derived from adipose mesenchymal stem cells (MSCs) after co-culture with CRC cell lines. Transwell assays showed that CAFs have stronger migration and invasion abilities than MSCs. In a nude mouse subcutaneous xenograft model, CAFs metastasized from the primary tumour to the lung and promoted the formation of CRC metastases. The expression of HIF-1α was upregulated when MSCs differentiated into CAFs. Inhibition of HIF-1α expression inhibited the migration and invasion of CAFs. Western blot and ChIP assays were used to identify the genes regulated by HIF-1α. HIF-1α regulated the migration and invasion of CAFs by upregulating miR-210 transcription. Bioinformatics analysis and luciferase reporter assays revealed that miR-210 specifically targeted the 3'UTR of VMP1 and regulated its expression. Downregulation of VMP1 enhanced the migration and invasion of CAFs. In vivo, inhibition of miR-210 expression in CAFs reduced the metastasis of CAFs and tumour cells. Therefore, the HIF-1α/miR-210/VMP1 pathway might regulate the migration and invasion of CAFs in CRC. Inhibition of CAF metastasis might reduce CRC metastasis.
Keywords: HIF-1α; cancer-associated fibroblasts; colorectal cancer; invasion; migration.
copyright: © 2021 Yang et al.
Conflict of interest statement
Conflicts of interest The authors declare no potential conflicts of interest.
Figures

Similar articles
-
Cancer-associated fibroblasts promote stemness maintenance and gemcitabine resistance via HIF-1α/miR-21 axis under hypoxic conditions in pancreatic cancer.Mol Carcinog. 2024 Mar;63(3):524-537. doi: 10.1002/mc.23668. Epub 2024 Jan 10. Mol Carcinog. 2024. PMID: 38197482
-
CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer.Mol Cancer. 2019 May 7;18(1):91. doi: 10.1186/s12943-019-1019-x. Mol Cancer. 2019. PMID: 31064356 Free PMC article.
-
The circular RNA circ-ERBIN promotes growth and metastasis of colorectal cancer by miR-125a-5p and miR-138-5p/4EBP-1 mediated cap-independent HIF-1α translation.Mol Cancer. 2020 Nov 23;19(1):164. doi: 10.1186/s12943-020-01272-9. Mol Cancer. 2020. PMID: 33225938 Free PMC article.
-
The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis.Cancers (Basel). 2021 Sep 21;13(18):4720. doi: 10.3390/cancers13184720. Cancers (Basel). 2021. PMID: 34572947 Free PMC article. Review.
-
The role of cancer-associated fibroblasts in the invasion and metastasis of colorectal cancer.Front Cell Dev Biol. 2024 Jul 30;12:1375543. doi: 10.3389/fcell.2024.1375543. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39139454 Free PMC article. Review.
Cited by
-
Non-Coding RNAs Implicated in the Tumor Microenvironment of Colorectal Cancer: Roles, Mechanisms and Clinical Study.Front Oncol. 2022 Apr 28;12:888276. doi: 10.3389/fonc.2022.888276. eCollection 2022. Front Oncol. 2022. PMID: 35574420 Free PMC article.
-
Expression of Selected miRNAs in Normal and Cancer-Associated Fibroblasts and in BxPc3 and MIA PaCa-2 Cell Lines of Pancreatic Ductal Adenocarcinoma.Int J Mol Sci. 2023 Feb 10;24(4):3617. doi: 10.3390/ijms24043617. Int J Mol Sci. 2023. PMID: 36835029 Free PMC article.
-
Prolyl 4-hydroxylase subunit beta (P4HB) could serve as a prognostic and radiosensitivity biomarker for prostate cancer patients.Eur J Med Res. 2023 Jul 22;28(1):245. doi: 10.1186/s40001-023-01215-2. Eur J Med Res. 2023. PMID: 37480146 Free PMC article.
-
Phase separation of DDX21 promotes colorectal cancer metastasis via MCM5-dependent EMT pathway.Oncogene. 2023 May;42(21):1704-1715. doi: 10.1038/s41388-023-02687-6. Epub 2023 Apr 7. Oncogene. 2023. PMID: 37029300 Free PMC article.
-
Establishment of cancer-associated fibroblasts-related subtypes and prognostic index for prostate cancer through single-cell and bulk RNA transcriptome.Sci Rep. 2023 Jun 3;13(1):9016. doi: 10.1038/s41598-023-36125-0. Sci Rep. 2023. PMID: 37270661 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 68:394-424. - PubMed
-
- Peinado H, Quintanilla M, Cano A (2003). Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem, 278:21113-21123. - PubMed
LinkOut - more resources
Full Text Sources
