RNAi-mediated silencing of PEX6 and GAS1 g enes of Fusarium oxysporum f. sp. lycopersici confers resistance against Fusarium wilt in tomato
- PMID: 34631344
- PMCID: PMC8455778
- DOI: 10.1007/s13205-021-02973-8
RNAi-mediated silencing of PEX6 and GAS1 g enes of Fusarium oxysporum f. sp. lycopersici confers resistance against Fusarium wilt in tomato
Abstract
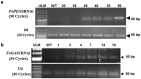
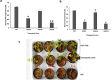
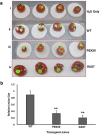
In the present study, we have explored the potential of the RNAi mediated silencing of genes encoding peroxisomal biogenesis factor and β-1,3-glucanosyltransferase in Fusarium oxysporum f. sp. lycopersici (Fol) to confer resistance to Fusarium wilt in transgenic tomato plants. The partial gene fragments from these genes were utilized independently to generate hairpin RNAi constructs in appropriate silencing vectors and used for Agrobacterium-mediated transformation of tomato. The presence of gene-specific siRNAs was confirmed by stem-loop RT-PCR analysis of selected transgenic tomato lines. Transgenic lines expressing gene-specific dsRNA displayed enhanced resistance to Fol with delayed development of disease symptoms. The survival rate of transgenic tomato lines after fungal infection was higher as compared to that of the untransformed tomato plants.
Supplementary information: The online version contains supplementary material available at 10.1007/s13205-021-02973-8.
Keywords: Fungal resistance; Fusarium oxysporum; Gene silencing; RNA interference; Tomato.
© King Abdulaziz City for Science and Technology 2021.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures

Similar articles
-
Host RNAi-mediated silencing of Fusarium oxysporum f. sp. lycopersici specific-fasciclin-like protein genes provides improved resistance to Fusarium wilt in Solanum lycopersicum.Planta. 2024 Mar 3;259(4):79. doi: 10.1007/s00425-024-04360-y. Planta. 2024. PMID: 38431538
-
Host-Induced Silencing of Pathogenicity Genes Enhances Resistance to Fusarium oxysporum Wilt in Tomato.Mol Biotechnol. 2017 Aug;59(8):343-352. doi: 10.1007/s12033-017-0022-y. Mol Biotechnol. 2017. PMID: 28674943
-
RNAi-mediated down-regulation of fasciclin-like proteins (FoFLPs) in Fusarium oxysporum f. sp. lycopersici results in reduced pathogenicity and virulence.Microbiol Res. 2022 Jul;260:127033. doi: 10.1016/j.micres.2022.127033. Epub 2022 Apr 18. Microbiol Res. 2022. PMID: 35487139
-
Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses.New Phytol. 2021 Oct;232(2):705-718. doi: 10.1111/nph.17436. Epub 2021 May 30. New Phytol. 2021. PMID: 33960431 Free PMC article.
-
Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity- A review.Saudi J Biol Sci. 2019 Nov;26(7):1315-1324. doi: 10.1016/j.sjbs.2019.06.002. Epub 2019 Jun 4. Saudi J Biol Sci. 2019. PMID: 31762590 Free PMC article. Review.
Cited by
-
Host RNAi-mediated silencing of Fusarium oxysporum f. sp. lycopersici specific-fasciclin-like protein genes provides improved resistance to Fusarium wilt in Solanum lycopersicum.Planta. 2024 Mar 3;259(4):79. doi: 10.1007/s00425-024-04360-y. Planta. 2024. PMID: 38431538
-
The essential role of fungal peroxisomes in plant infection.Mol Plant Pathol. 2022 Jun;23(6):781-794. doi: 10.1111/mpp.13180. Epub 2022 Jan 10. Mol Plant Pathol. 2022. PMID: 35001508 Free PMC article. Review.
-
Groundbreaking Technologies and the Biocontrol of Fungal Vascular Plant Pathogens.J Fungi (Basel). 2025 Jan 18;11(1):77. doi: 10.3390/jof11010077. J Fungi (Basel). 2025. PMID: 39852495 Free PMC article. Review.
-
Harnessing RNA interference for the control of Fusarium species: A critical review.Mol Plant Pathol. 2024 Oct;25(10):e70011. doi: 10.1111/mpp.70011. Mol Plant Pathol. 2024. PMID: 39363756 Free PMC article. Review.
References
-
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15.
LinkOut - more resources
Full Text Sources
Miscellaneous

